High-performance liquid chromatography separation and determination method for related impurities in fosfomycin trometamol raw material drug and preparations thereof and application thereof
A technology of fosfomycin tromethamine and high performance liquid chromatography, which is applied in the field of medicine, can solve the problems affecting the impurity composition of fosfomycin tromethamine preparations, lack of structure confirmation and detection, and high detection cost, and achieve separation The detection method is simple and easy, the retention time changes greatly, and the effect of overcoming the rapid loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
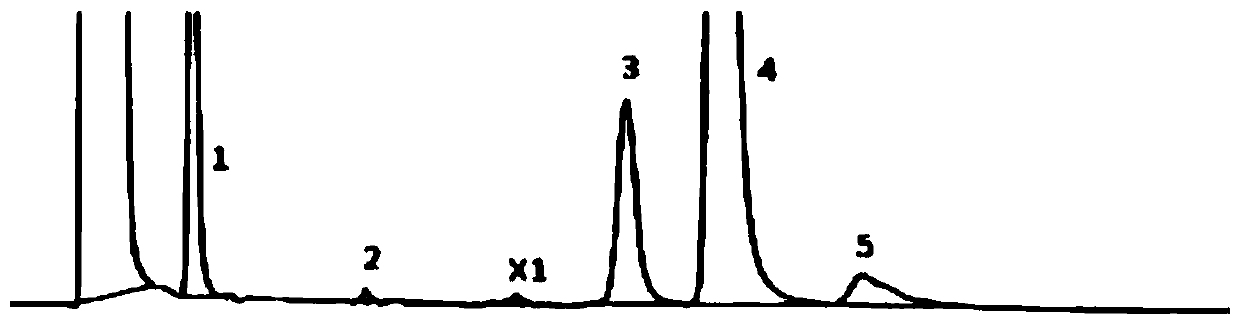
Embodiment 1
[0067] (1) Chromatographic conditions:
[0068] Column: Agilent Zorbax NH 2 (5μm, 4.6mm×250mm)
[0069] Column temperature: 30°C
[0070] Mobile phase: 20mmol / L phosphate buffer (potassium dihydrogen phosphate)-methanol (70:30)
[0071] Flow rate: 1.0ml / min
[0072] Detection: differential detector, detection temperature: 35°C
[0073] Injection volume: 10μl
[0074] (2) Sample solution preparation:
[0075] System suitability solution: take 0.3g of fosfomycin trometamol (batch number: Y18011607), wet it with 10μl of water, heat at 80°C for 12 hours, add mobile phase to dissolve and dilute to 10ml, shake well, and use it as solution A; Take another 0.3g of this product, dissolve it with solution A and dilute to 2.5ml, as a system suitability solution.
[0076] Sample solution: before using the new system, get fosfomycin tromethamine granules (lot number: 342414) in an appropriate amount, accurately weighed, add mobile phase to dissolve and quantitatively dilute into abo...
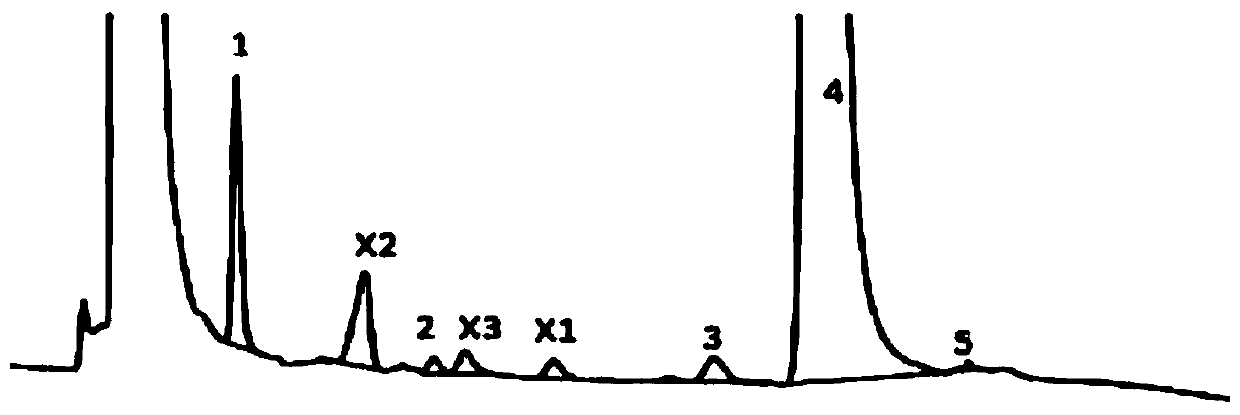
Embodiment 2
[0085] (1) Chromatographic conditions:
[0086] Column: Osaka Soda Capcell Pak NH 2 (5μm, 4.6×250mm)
[0087] Column temperature: 40°C
[0088] Mobile phase: 80mmol / L phosphate buffer (dipotassium hydrogen phosphate)-methanol (75:25)
[0089] Flow rate: 0.8ml / min
[0090] Detection: differential detector, detection temperature: 50°C
[0091] Injection volume: 1μl
[0092] (2) Sample solution preparation:
[0093] System suitability solution: Take 0.3g of fosfomycin trometamol (batch number: Y18011607), moisten it with 60μl of water, heat at 60°C for 12 hours, add mobile phase to dissolve and dilute to 20ml, shake well, and use it as solution A; Take another 0.3g of this product, dissolve it with solution A and dilute to 5ml, as a system suitability solution.
[0094] Sample solution: just use the new system, get fosfomycin tromethamine granules (lot number: 342414) in an appropriate amount, accurately weighed, add mobile phase to dissolve and quantitatively dilute into ...
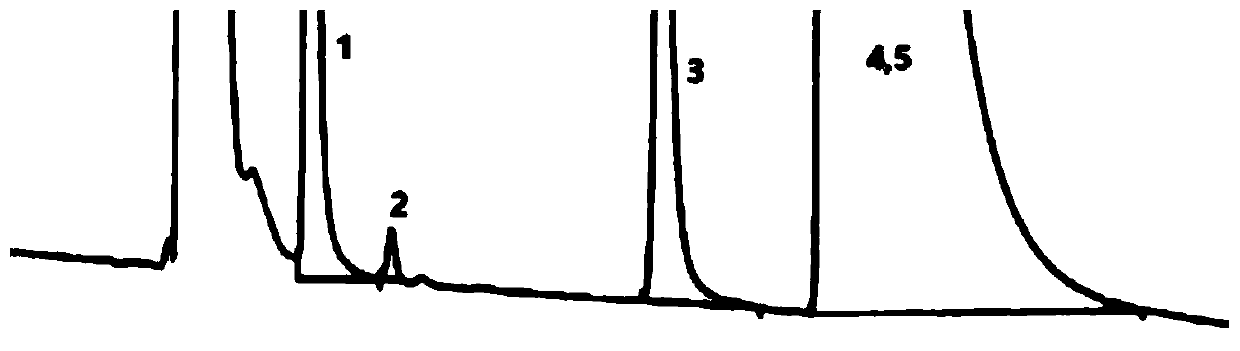
Embodiment 3
[0097] (1) Chromatographic conditions:
[0098] Column: Phenomenex Luna-NH 2 (5μm, 4.6×250mm)
[0099] Column temperature: 20°C
[0100] Mobile phase: 50mmol / L phosphate buffer (potassium phosphate)-methanol (50:50)
[0101] Flow rate: 1.2ml / min
[0102] Detection: differential detector, detection temperature: 15°C
[0103] Injection volume: 100μl
[0104] (2) Sample solution preparation:
[0105] System suitability solution: Take 0.3g of fosfomycin trometamol powder (batch number: 20150501), wet it with 300μl of water, heat at 40°C for 12 hours, add mobile phase to dissolve and dilute to 50ml, shake well, and use it as solution A ; Take another 0.3g of this product, dissolve it with solution A and dilute it to 10ml, as a system suitability solution.
[0106]Sample solution: before using the new system, get fosfomycin tromethamine powder (batch number: 20150501) in an appropriate amount, accurately weighed, add mobile phase to dissolve and quantitatively dilute into abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com