Analytic method of omeprazole related substance
An analysis method and related substance technology, applied in the analysis of materials, material separation, instruments, etc., can solve the problems of low sensitivity, less detected impurities, harsh chromatographic conditions, etc., and achieve the effect of high sensitivity, effective method and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
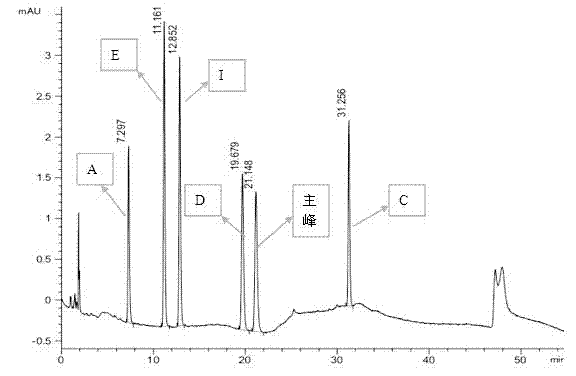
[0021] Example 1 High performance liquid chromatography analysis of omeprazole and several impurities
[0022] Several related substances are: A, D, E, C, I, F.
[0023] Instruments and analysis conditions - Agilent 1100, chromatographic workstation, automatic sampler, column oven. The chromatographic column is Agilent XDB-C18 (4.6mm*150mm, 5um), the detection wavelength is 280 nm, the flow rate is 1.0 ml / min, the column temperature is 30 ℃, the organic phase is acetonitrile, and the aqueous phase is phosphate buffer with pH 7.6 , the gradient elution method is:
[0024]
[0025] Experimental steps:
[0026] Accurately weigh an appropriate amount of omeprazole and several impurities (A, C, D, E, I), dissolve them with a diluent and constant volume, and make a mixed solution containing omeprazole and related impurities as the test solution . Perform high-performance liquid phase analysis according to the above conditions, inject 20 ul, see the attached manual for ...
Embodiment 2
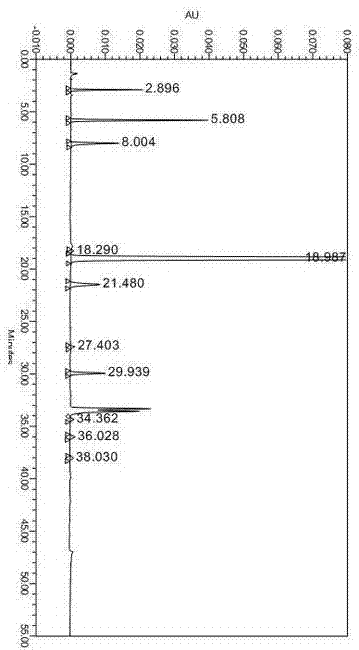
[0028] Embodiment two Methodological investigation of the analytical method of the present invention
[0029] Examination of specificity: other excipients without omeprazole were prepared for destruction, and the excipients did not interfere with the determination of omeprazole-related substances, which proved that their specificity was good.
Embodiment
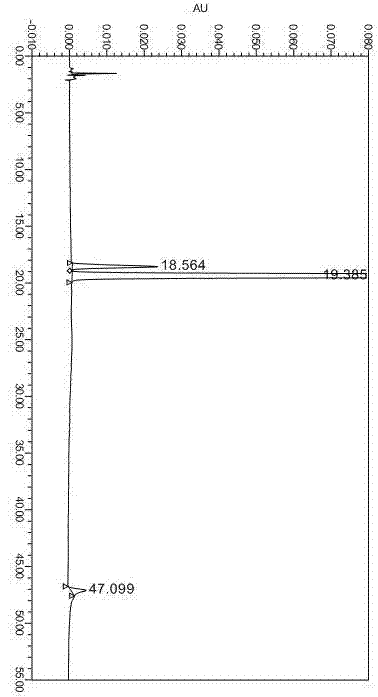
[0030] Embodiment Analytical method Stability test
[0031] In order to better examine the specificity and stability of this method, acid, alkali, oxidation and water damage tests were designed.
[0032] Instruments and analysis conditions - Water 2695 chromatographic workstation, automatic sampler, column thermostat. The chromatographic column is Agilent XDB-C18 (4.6mm*150mm, 5um), the detection wavelength is 280nm, the flow rate is 1.0ml / min, the column temperature is 30 ℃, the organic phase is acetonitrile, and the aqueous phase is phosphate buffer with pH7.6 , the gradient elution method is:
[0033]
[0034] Acid destruction test: Weigh 20mg of omeprazole sample into a 100ml volumetric flask, add 5ml of 0.1mol / L hydrochloric acid solution, let it destroy for 2min, add 0.1mol / L NaOH solution to neutralize, dilute to volume with diluent, shake well, After centrifugation, inject into the liquid chromatograph, inject 20ul of the sample, record the chromatogram, see...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com