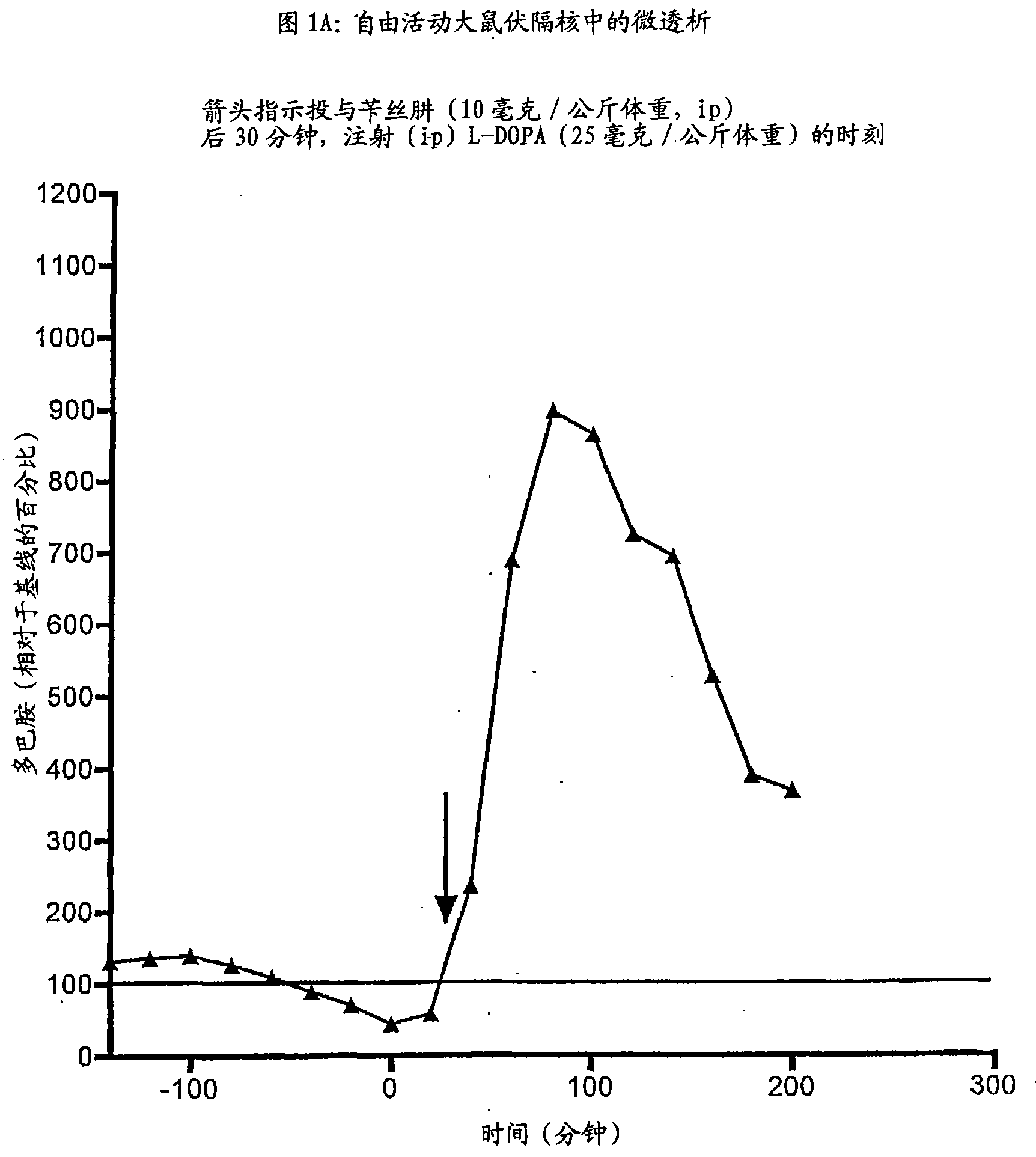
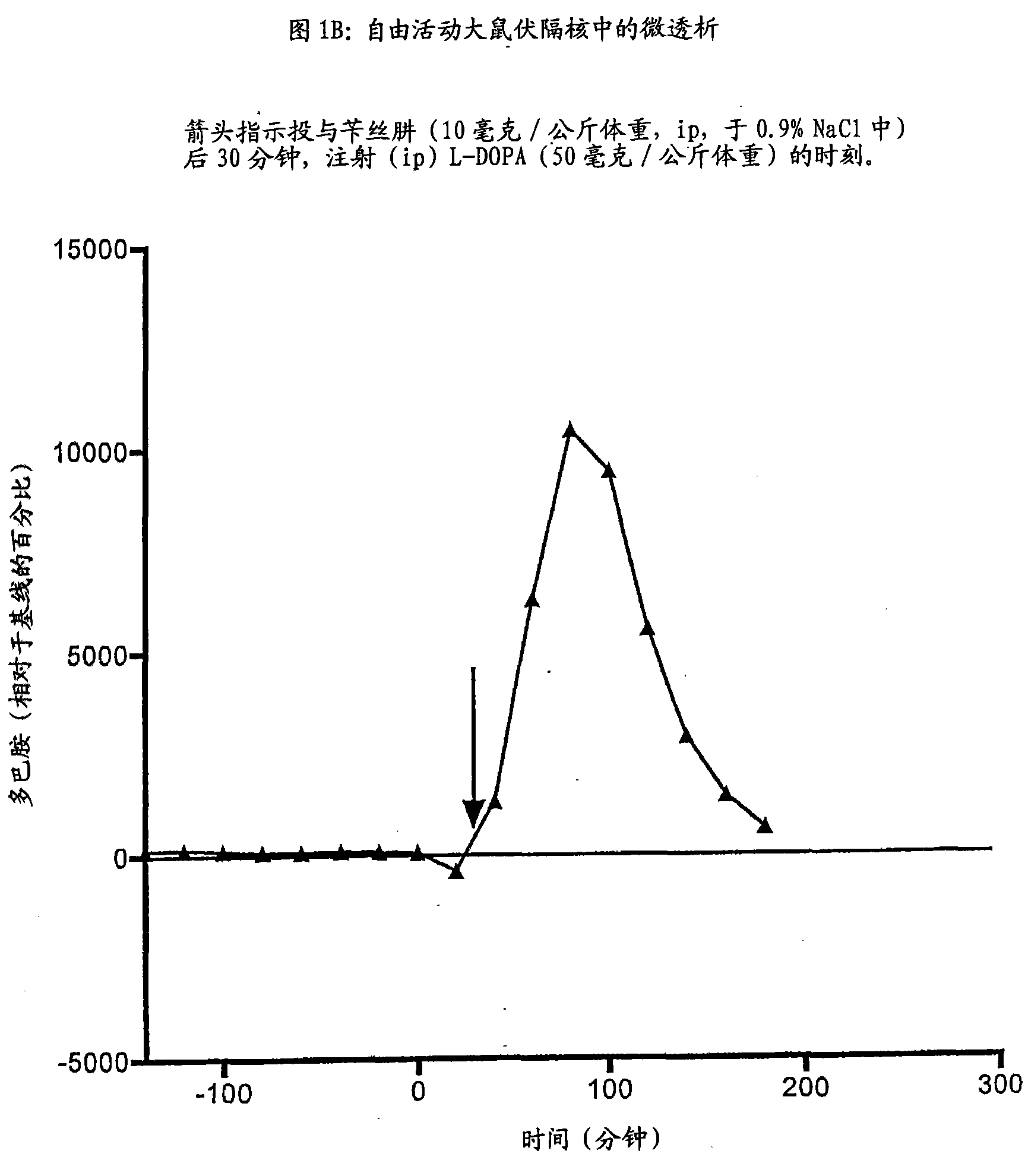
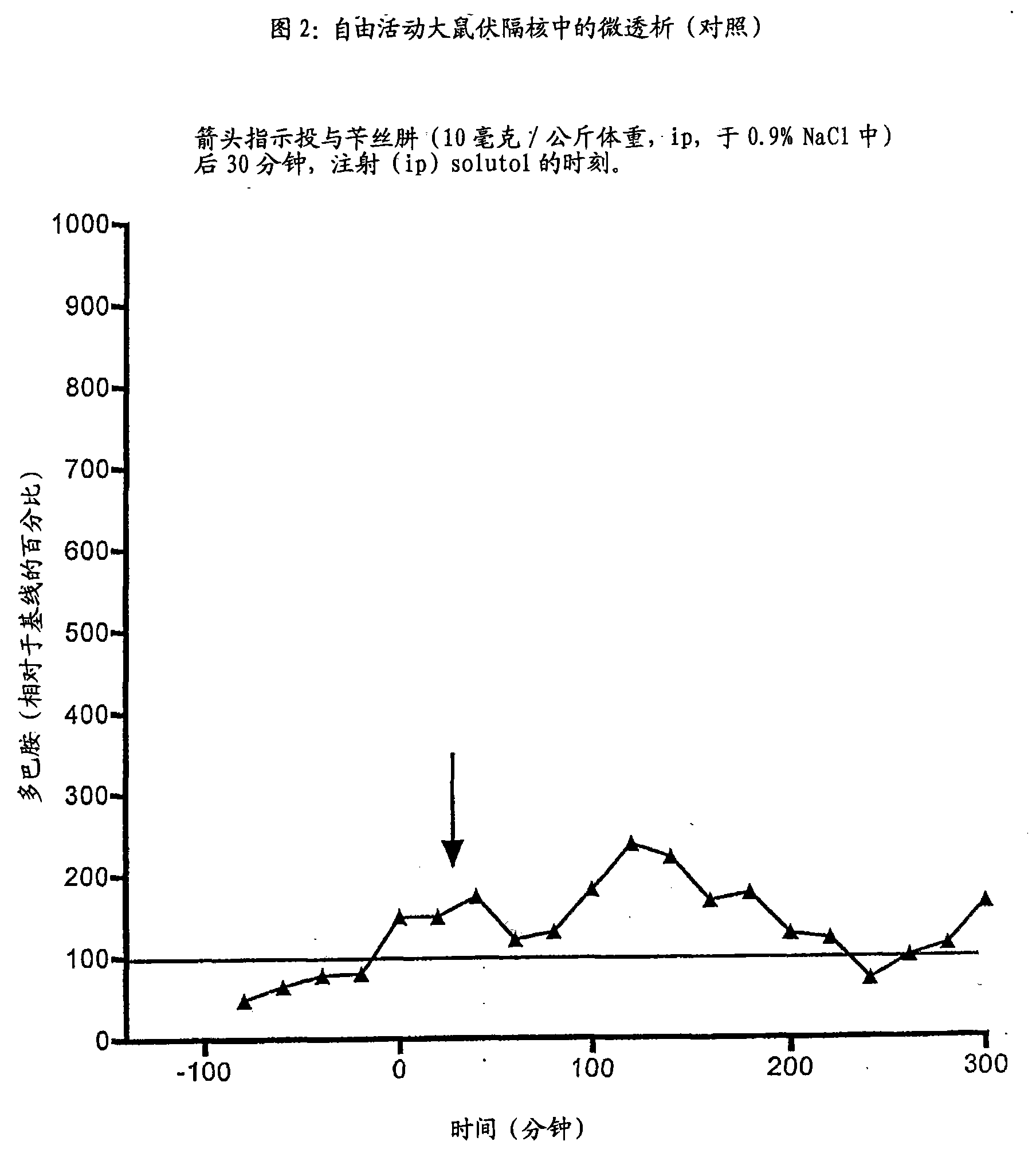
Derivate von dihydroxyphenylalanin
A technology of residues and compounds, applied in the field of dihydroxyphenylalanine derivatives, can solve problems such as the inability to ensure the formation of a sufficient amount of the chemical messenger dopamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0095] Example 1: Synthesis of O,O'-Diacetyl-L-DOPA-ethylene glycol lipoate (Diacetyl-DOPA-ethylene glycol lipoate; Compound 1)
[0096]
[0097] Lipoic acid is converted to lipoic acid monoethylene glycol ester using excess ethylene glycol and DCC. Conversion of L-DOPA to Fmoc-L-DOPA by Fmoc-succinimide, and acetylation of Fmoc-L-DOPA to N-Fmoc via acetic anhydride under Schotten-Baumann conditions -O,O'-diacetyl-L-DOPA. The coupling product N-Fmoc-O,O'-diacetyl-L-DOPA ethylene glycol-racemic (rac)-lipoate was obtained by converting the above lipoic acid monoethylene glycol ester with DDC. The Fmoc protecting group was cleaved via tetrabutylammonium fluoride in DMF.
[0098] Purity (HPLC): 81-85%, clear yellow oil
[0099] 13 C-NMR (100.6MHz, d 4 - Methanol), δ (ppm):
[0100] 20.47; 25.71; 29.74; 34.76; 35.68; 39.33; 41.07; 41.27; 57.51;
example 2
[0101] Example 2: Synthesis of O,O'-diacetyl-L-DOPA-(R,S)-lipoic acid amide (Diacetyl-DOPA-lipoic acid amide; Compound 2)
[0102]
[0103] O, O'-diacetyl-L-DOPA-rac-lipoic acid amide is obtained by reacting L-DOPA-rac-lipoic acid amide with acetic anhydride under mild alkaline reaction conditions.
[0104] Purity (HPLC)>95%, clear yellow oil
[0105] 13 C-NMR (100.6, CDCl 3 ), δ(ppm):
[0106] 20.65; 25.16; 28.67; 34.50; 35.99; 36.48; 38.43; 40.17; 52.74;
example 3
[0107] Example 3: Synthesis of L-DOPA-(D,L)-lipoic acid amide (DOPA-lipoic acid amide; compound 3)
[0108]
[0109] L-DOPA-rac-ac- Lipoic acid amide. Lipoyl chloride is obtained from lipoic acid and oxalyl chloride, and lipoic acid succinimide ester is obtained from lipoic acid, hydroxysuccinimide and DCC.
[0110] Purity (HPLC) 97%, yellow highly viscous oil
[0111] 13 C-NMR (100.6, d 4 - Methanol), δ (ppm):
[0112] 26.58; 29.60; 35.69; 36.60; 37.83; 39.31; 46.23; 55.00; 57.43;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com