Applications of exosome from mesenchymal stem cells
A technology of mesenchymal stem cells and exosomes, applied in medical preparations containing active ingredients, sensory diseases, unknown raw materials, etc., can solve the problems of clinical application of tumorigenic risks, low survival rate of transplantation, difficulties in storage and transportation, etc., to achieve Improve retinal function, reduce inflammation, and reduce retinal damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
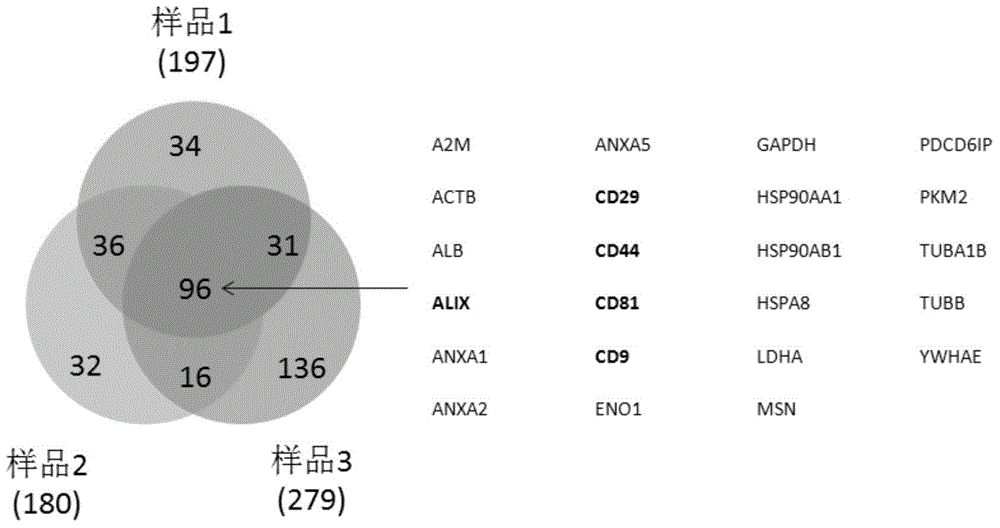
[0044] Isolation, purification and identification of exosomes derived from human umbilical cord mesenchymal stem cells (hucMSC)
[0045] 1. A method for separating and purifying exosomes derived from human umbilical cord mesenchymal stem cells, comprising the steps of:
[0046] (1) Isolation and culture of human umbilical cord mesenchymal stem cells: fresh umbilical cords of sterile newborns were taken, washed repeatedly with phosphate buffer saline (PBS), and cut into tissue pieces with a diameter of about 1-2 mm; treated with type 2 collagenase and After trypsin digestion, centrifuge the supernatant, take the cell pellet and put it into a culture bottle, use DMEM / F12 medium containing 10% fetal bovine serum, 5% CO 2 , Culture in saturated humidity at 37°C; remove non-adherent cells, and subculture after 80% fusion of adherent cells with 0.25% trypsin;
[0047] (2) Collection of conditioned medium for mesenchymal stem cells: take 3-5 generations of mesenchymal stem cells and...
Embodiment 2
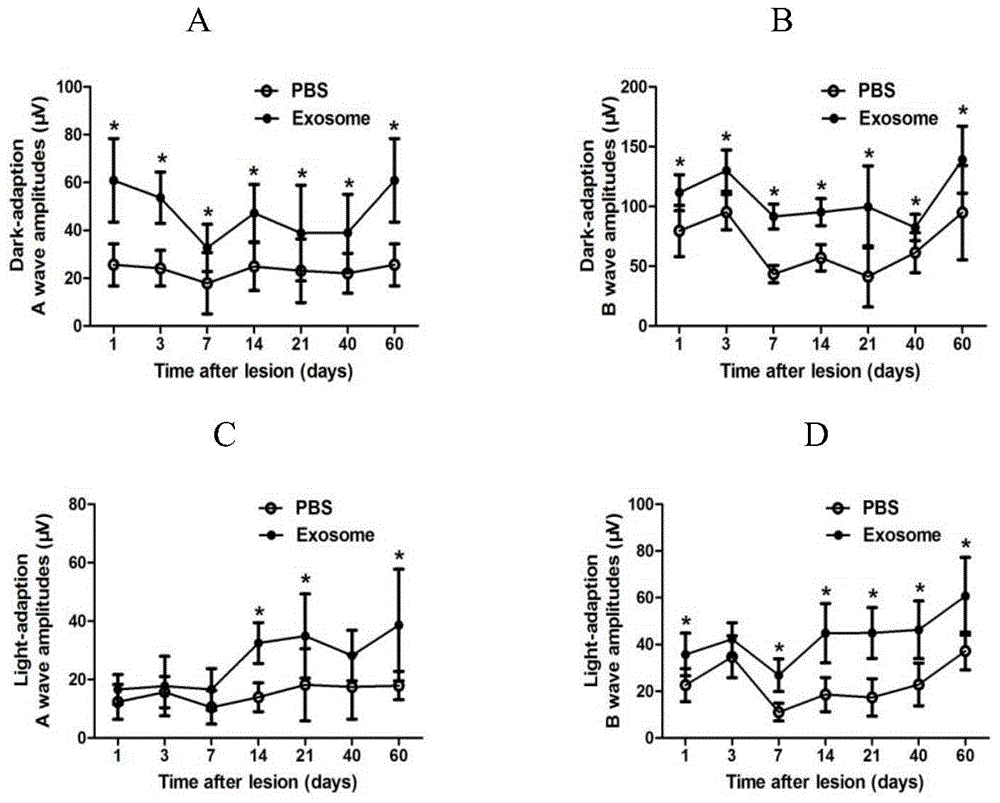
[0052] Example 2: Observation of curative effect of hucMSC-exosome on mouse retinal injury model
[0053] (1) Model establishment: 6-8 weeks old healthy female C57BL / 6 mice of SPF grade (body weight 15-18 g) were included in the experiment after examination confirmed that the refractive interstitium was clear and the fundus was normal. The husbandry practices followed the Society for Research in Vision and Ophthalmology Standards for the Care and Use of Animals in Ophthalmology and Vision Research. The mice were randomly divided into normal group, treatment group and injury control group. The mice in the normal group did not receive any treatment. The mice in the treatment group and the injury control group were anesthetized by intraperitoneal injection of 5% chloral hydrate at a dose of 500 mg / kg, and after the right eye was dilated with compound tropicamide, laser light was performed using a multi-wavelength krypton ion laser machine NOVUSOMNI (Coherent, USA). Condensation...
Embodiment 3
[0061] Example 3: Observation of curative effect of hucMSC-exosome on experimental autoimmune uveitis model in rats
[0062] (1) Model establishment: 6-8 week-old female Lewis rats, 30 μg of interphotoreceptor retinoid-binding protein (IRBP) R16 polypeptide fragment dissolved in phosphate buffered saline (PBS) and an equal volume containing 2.5 mg / ml Mycobacterium tuberculosis The complete Freund's adjuvant (CFA) of H37Ra was mixed in equal volume, and the three-way valve was fully emulsified. 0.2ml was taken from each rat and subcutaneously injected into one hind paw to establish the EAU model.
[0063] (2) Treatment method and grouping: Lewis rats were randomly divided into exosome treatment group and model control group with 6 rats after EAU modeling. The exosome treatment group was injected with 50 μl of PBS containing 30 μghucMSC-exosome daily in the juxtabulbar sub-tenon capsule from the initial stage of the disease for 7 consecutive days, and the corresponding model co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com