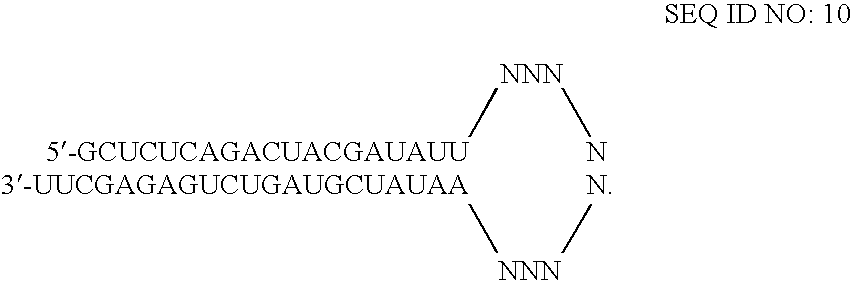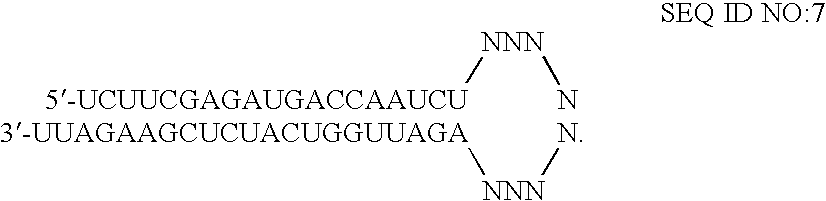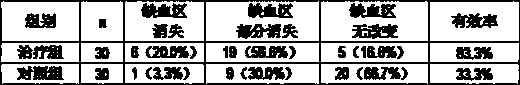Patents
Literature
42 results about "Retinal ischemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Retinal ischemia is an important possible aftereffect of a central retinal vein occlusion (CRVO). “Ischemia” is a term used to describe a tissue whose blood supply has been reduced to an insufficient level.
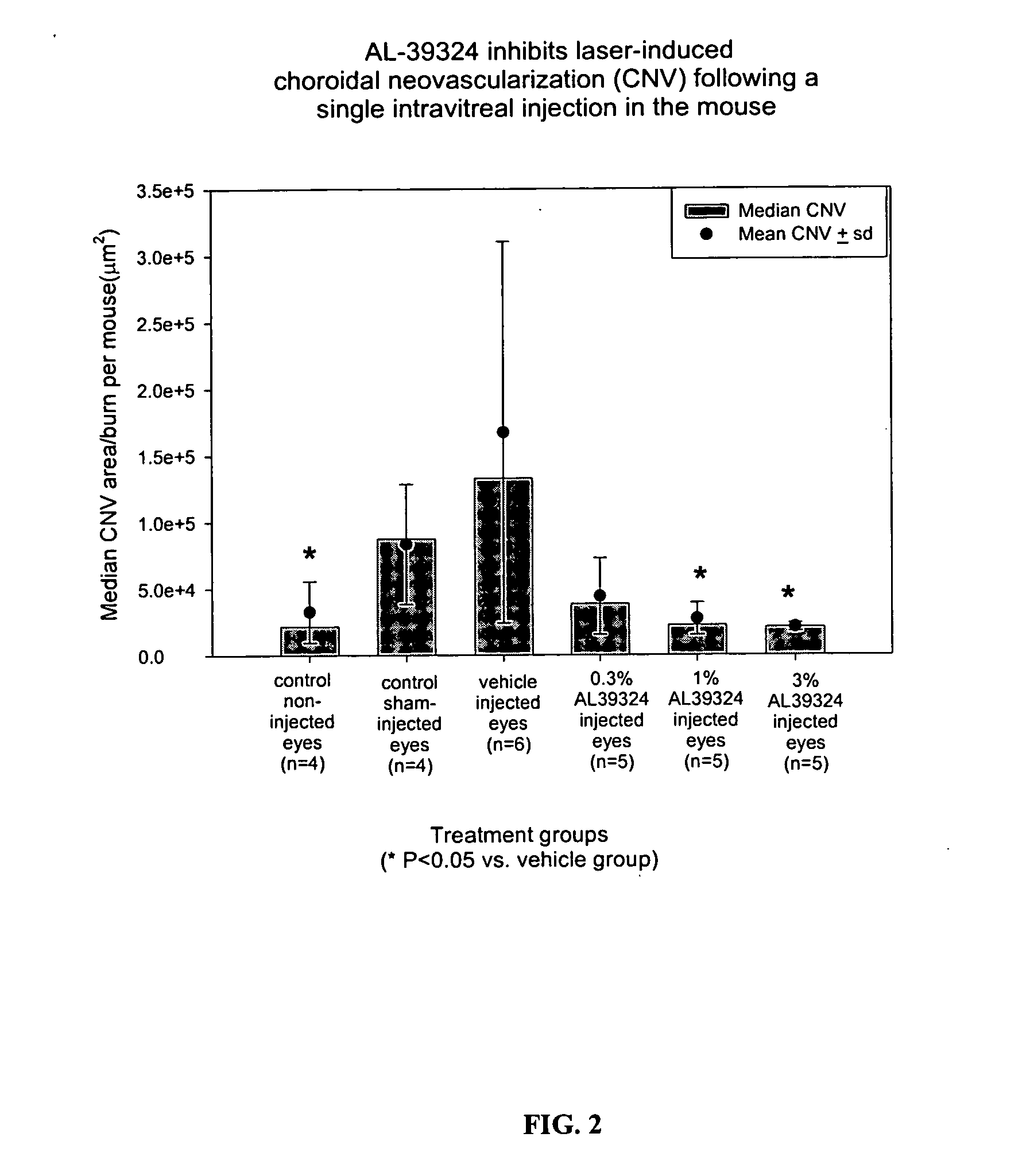
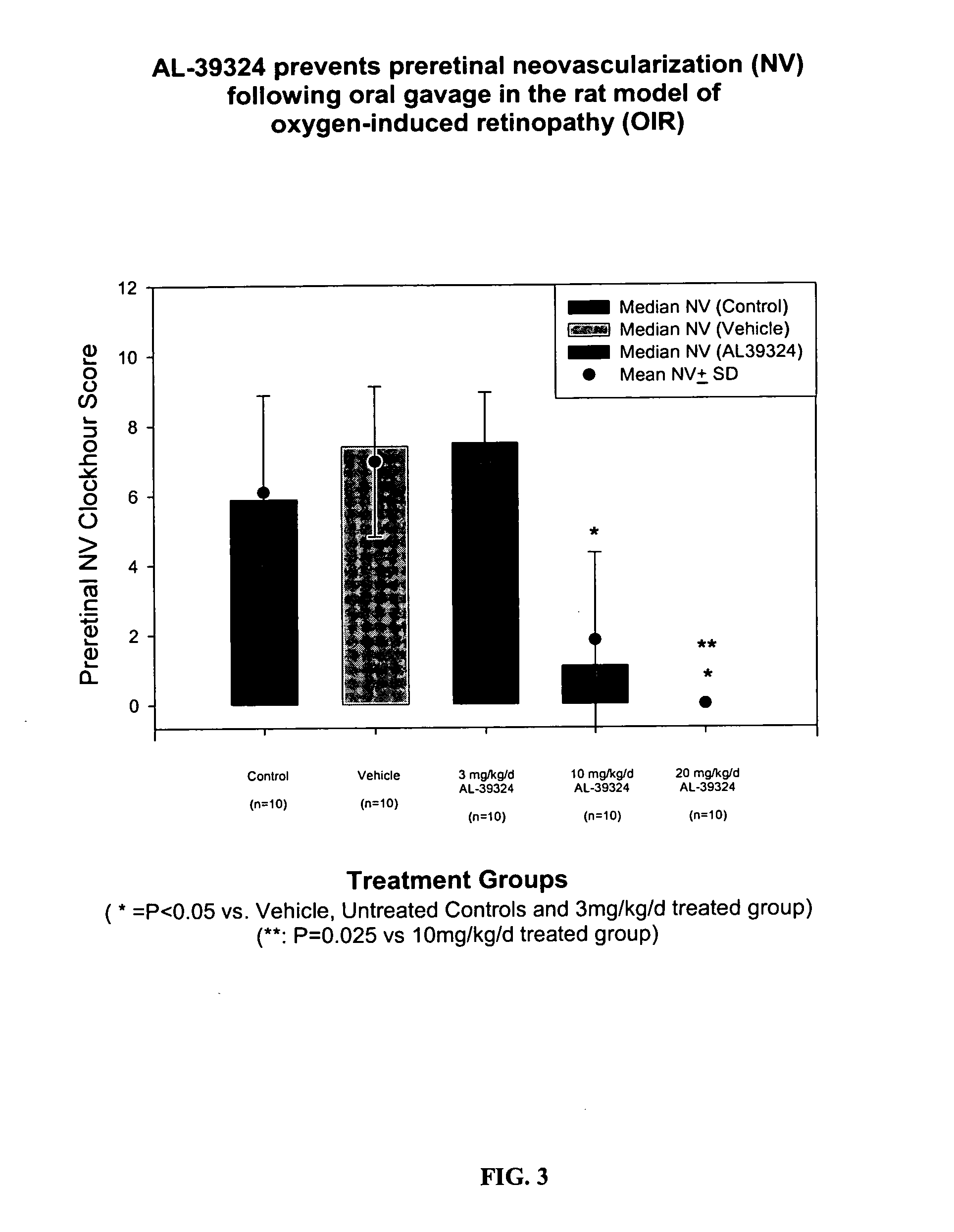
Methods for treating ocular angiogenesis, retinal edema, retinal ischemia, and diabetic retinopathy using selective RTK inhibitors
InactiveUS20060189608A1Highly potent and efficacious inhibitionBiocideSenses disorderDiseaseReceptor tyrosine kinase inhibitor
The present invention provides compositions and methods for treating ocular neovascularization, angiogenesis, retinal edema, diabetic retinopathy, and / or retinal ischemia in order to prevent the loss of visual acuity associated with such conditions. More specifically, the present invention provides compositions containing receptor tyrosine kinase (RTK) inhibitors having unique binding profiles and their use in treating ocular disorders.
Owner:NOVARTIS AG
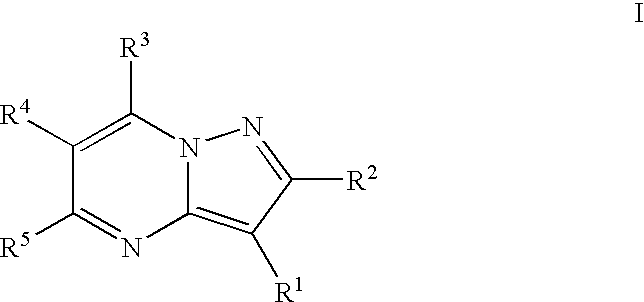
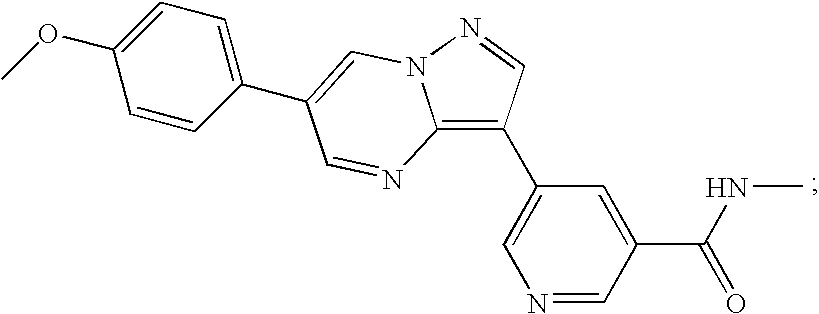
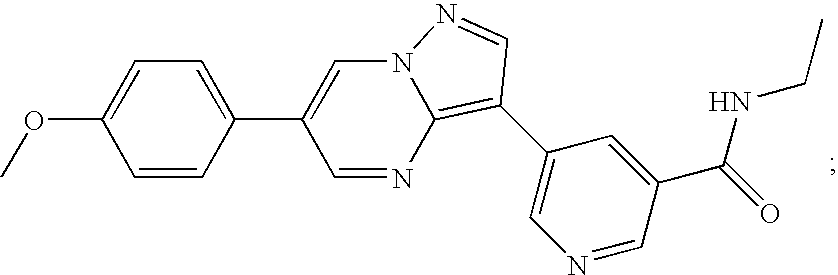
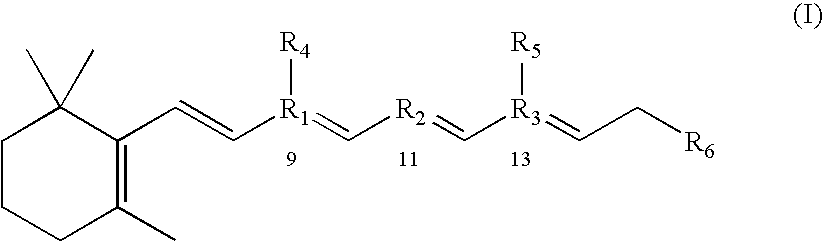
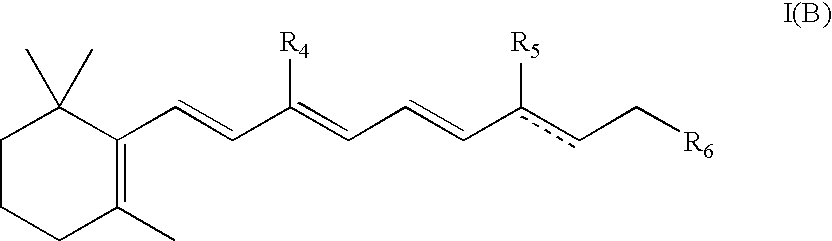
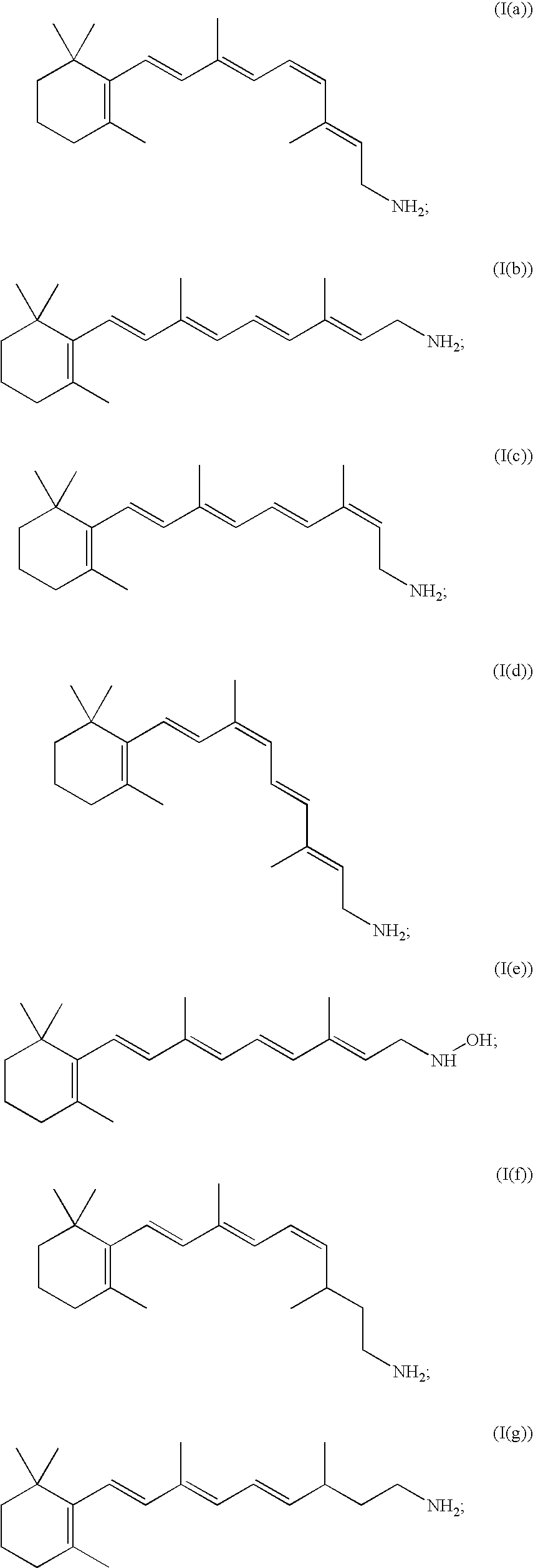
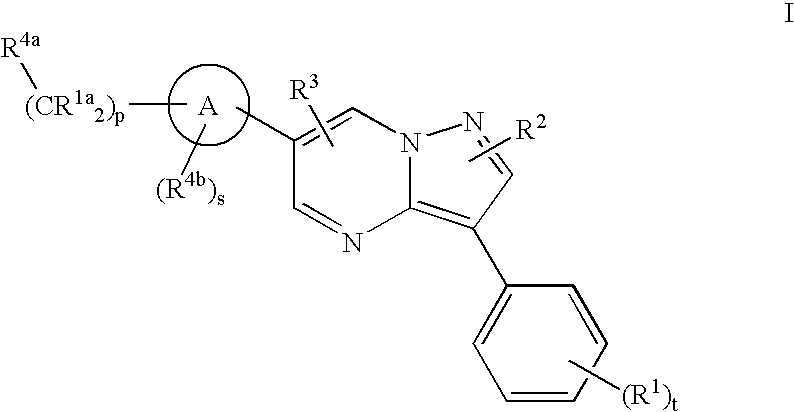
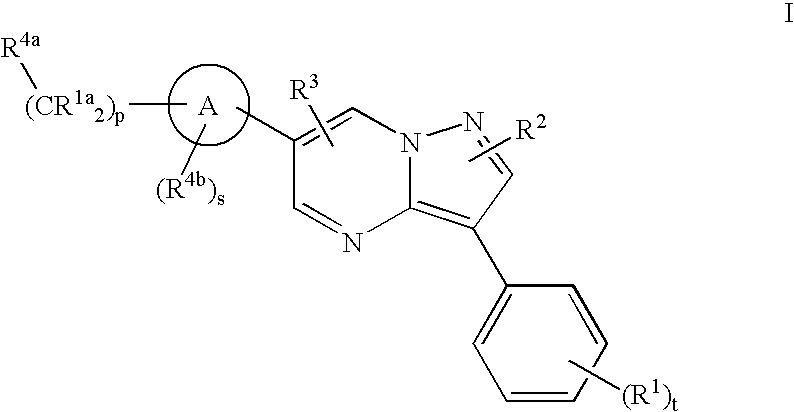
Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Prevention and treatment of retinal ischemia and edema
InactiveUS20080019977A1Inhibit bindingAvoid interactionBiocideOrganic active ingredientsCell adhesionOphthalmology
The present invention relates to methods of treating retinopathy, retinal ischemia and / or retinal edema comprising administering an integrin or integrin subunit antagonist, leukocyte adhesion-inducing cytokine antagonist or growth factor antagonist, a selectin antagonist or adhesion molecule antagonist.
Owner:CHILDRENS MEDICAL CENT CORP
Tyrosine kinase inhibitors
InactiveUS20060025426A1Organic active ingredientsBiocideDiabetic retinopathyTyrosine-kinase inhibitor
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
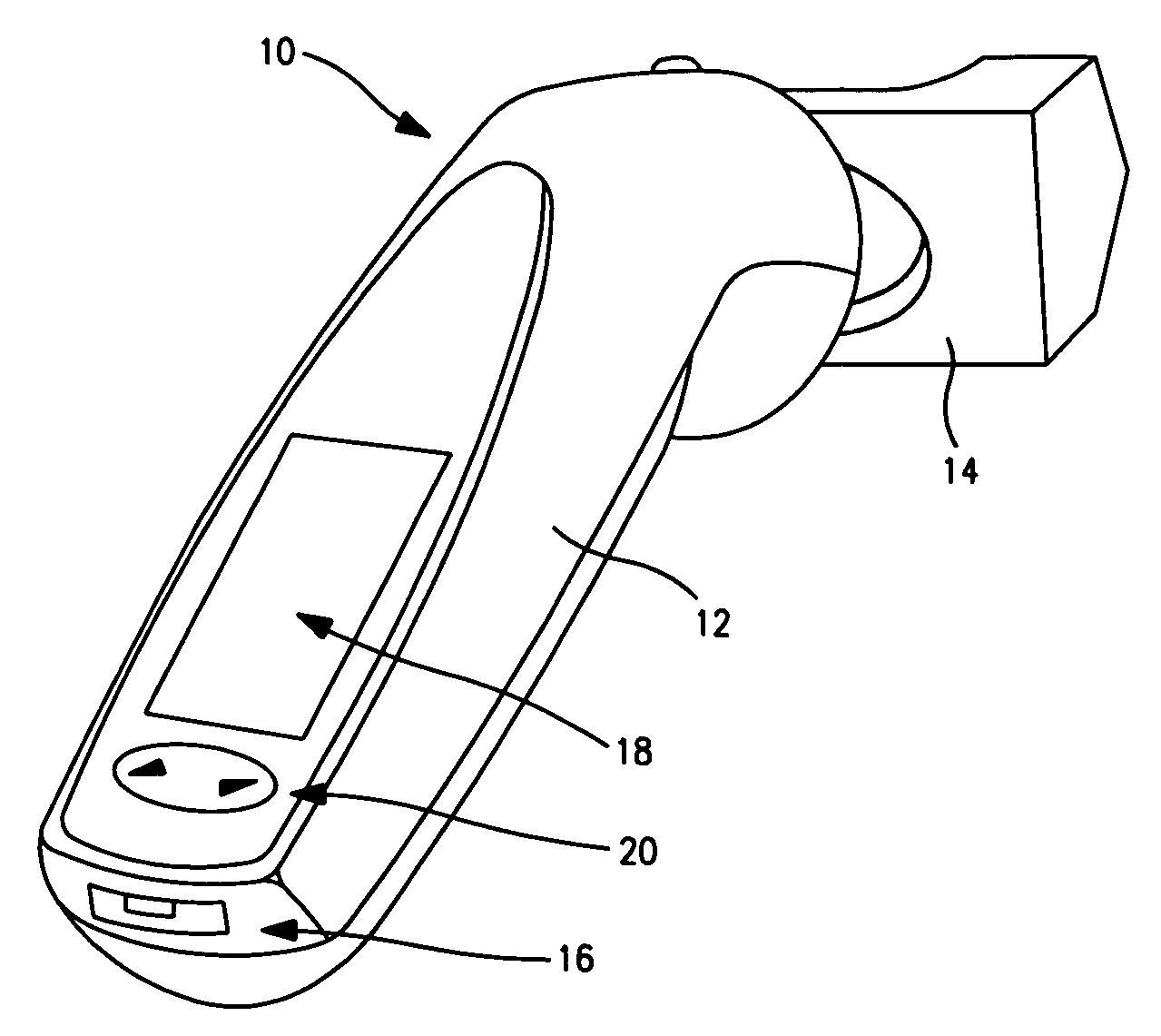
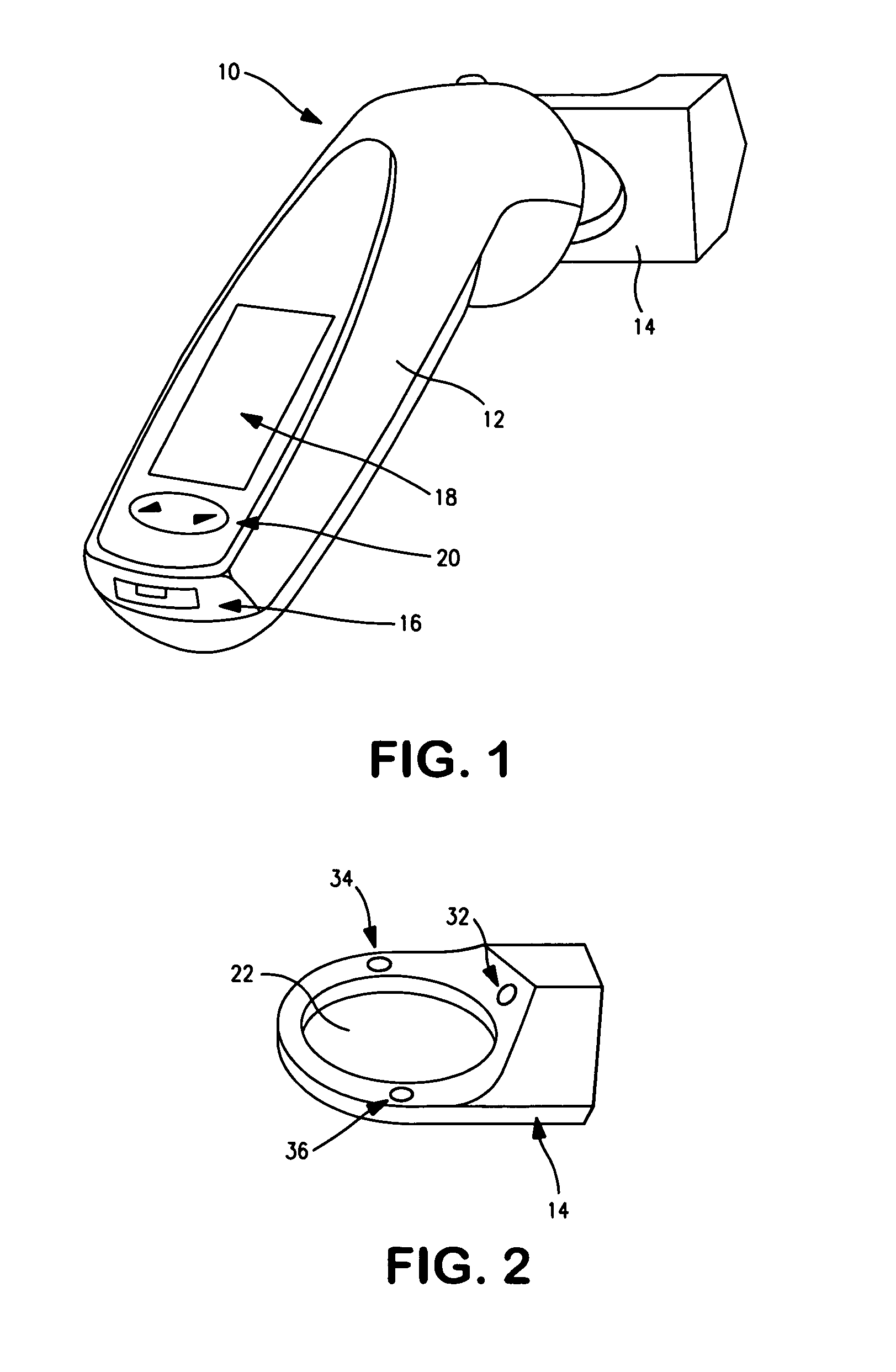
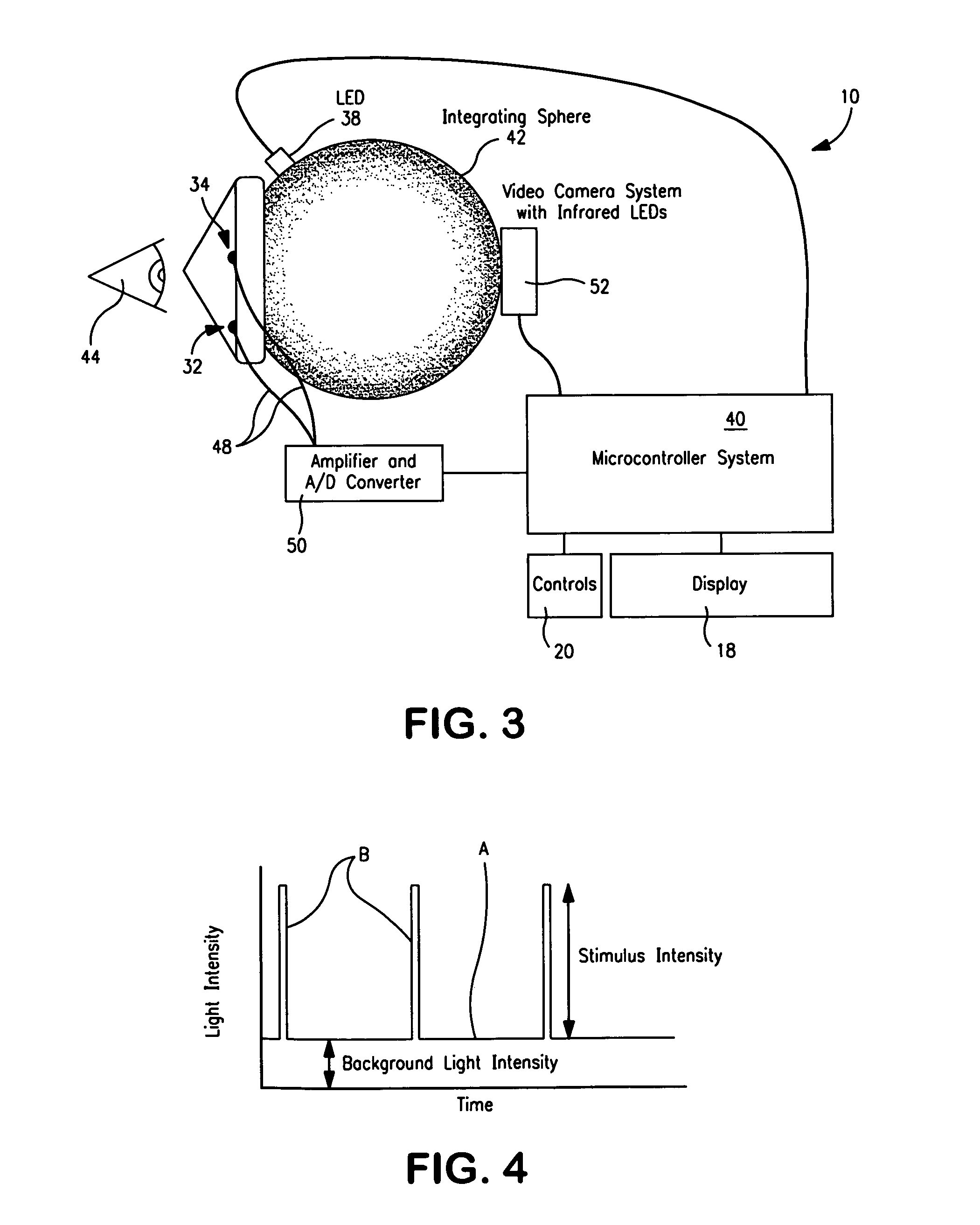
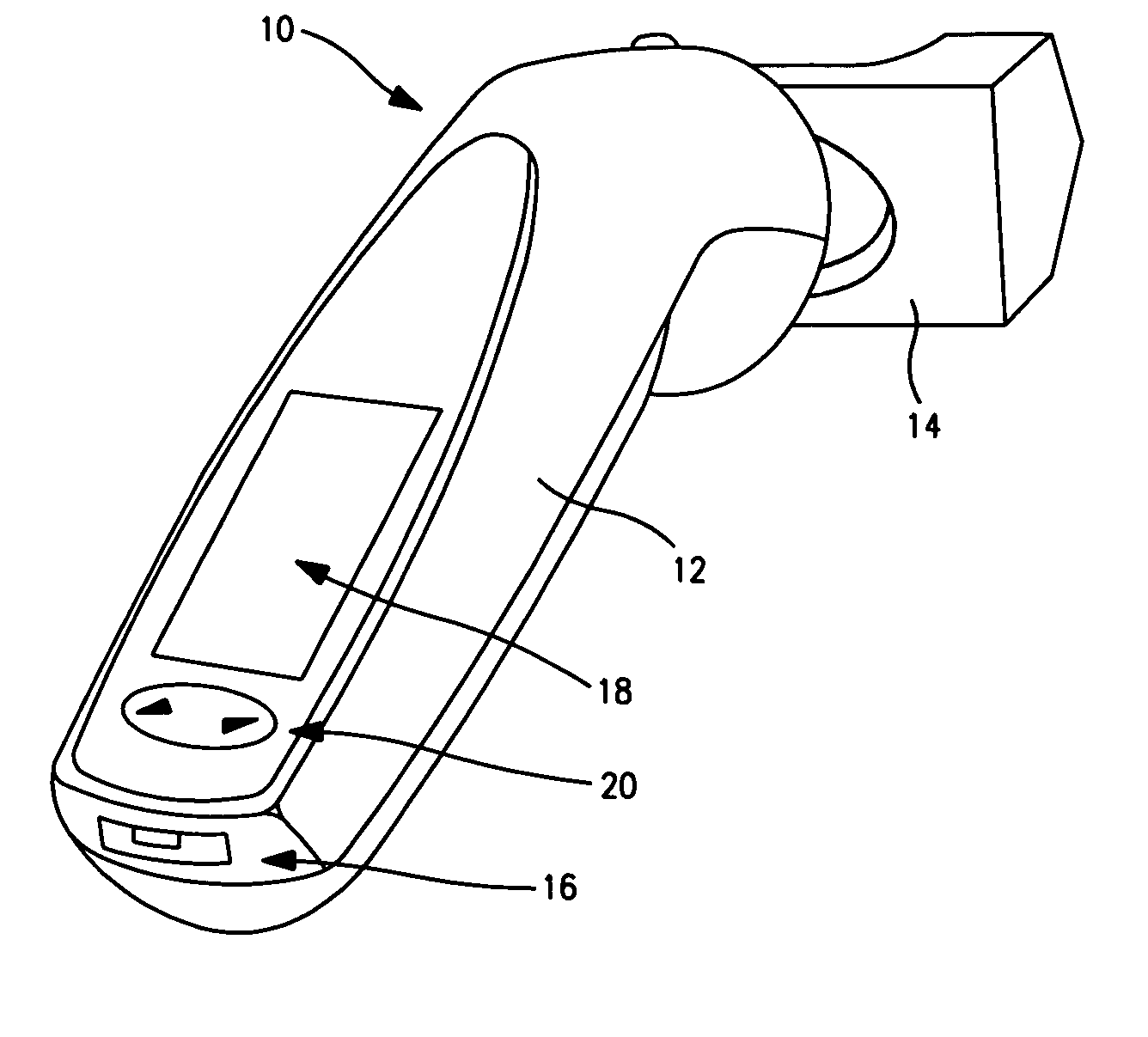
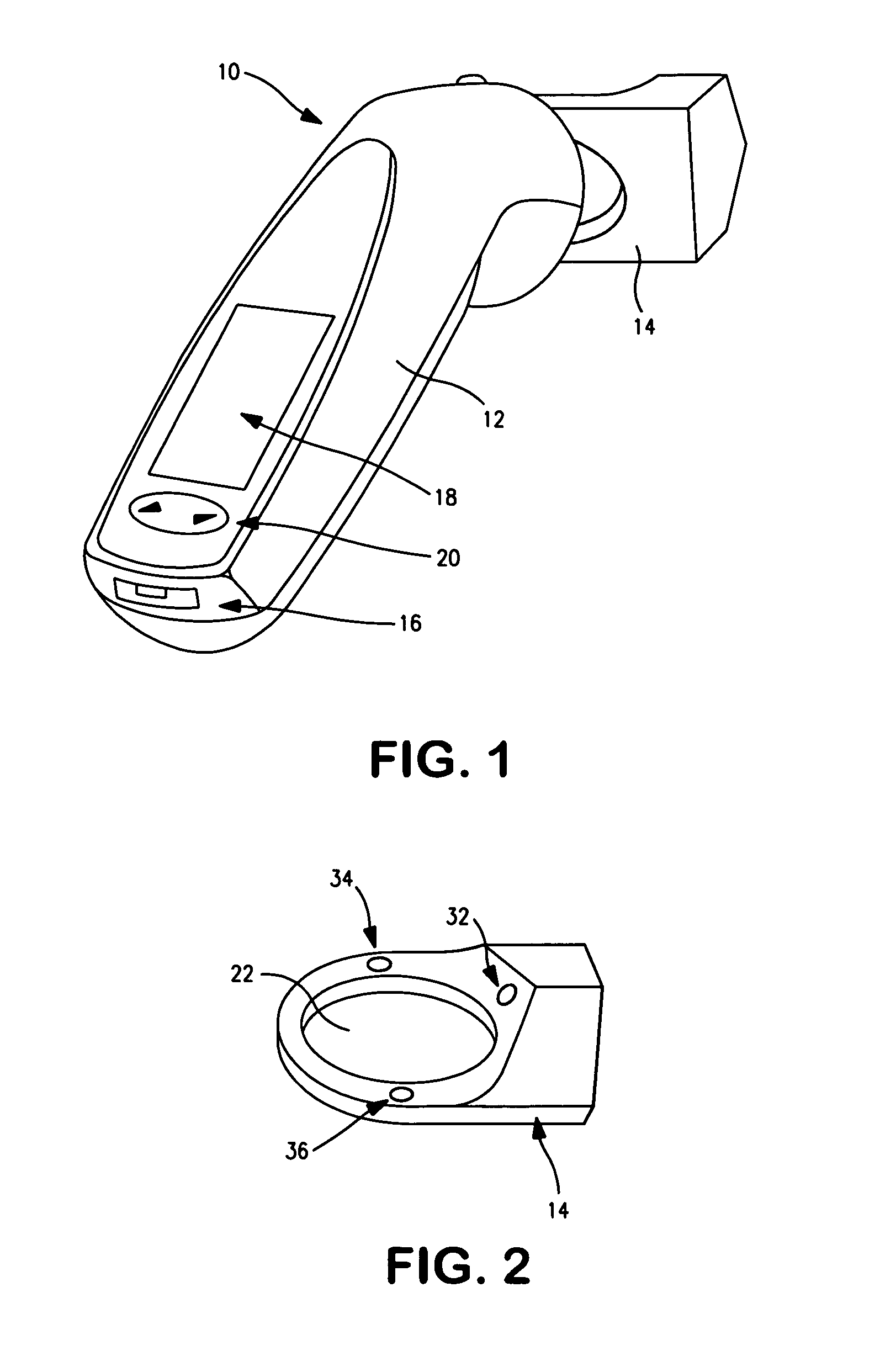
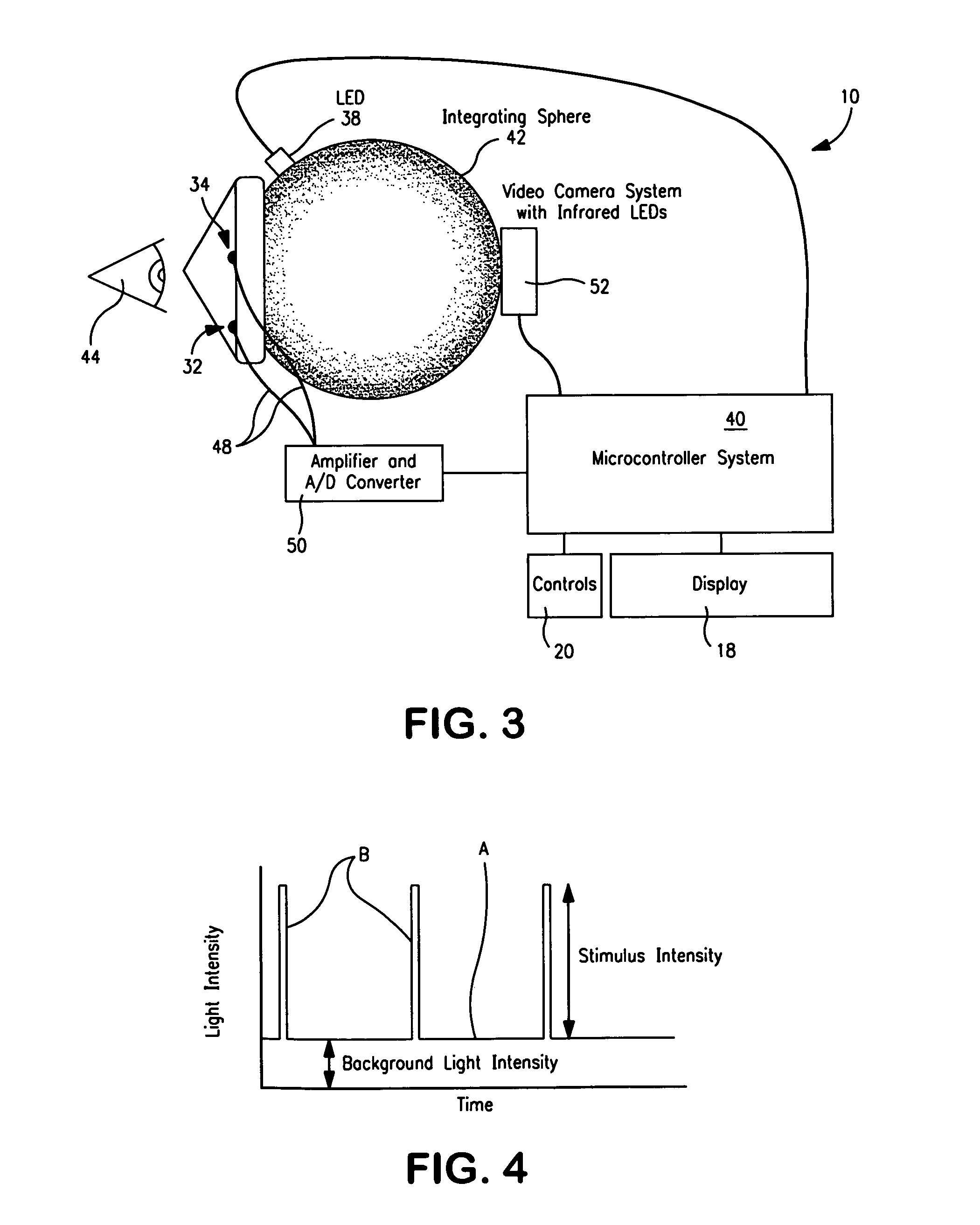
Device to monitor retinal ischemia
A battery powered, hand held device to determine of there is significant retinal ischemia in the eye of a patient. The device detects the consequences of impaired blood flow in the eye of the patient and has a light source for emitting a light and a diffuser or diffuse spheroidal reflector that redirects the light from the light source toward the patient's eye. A set of electrodes contact the patients skin proximate to the eye and receives an electrical signal representing the eye's electrical response to the light stimulus. A microcontroller interprets the electrical signal sensed by the electrodes by using an algorithm to determine the degree of retinal ischemia of the patient. In one embodiment, there is a control that establishes the intensity of the light stimulus by measuring and using the area of the pupil.
Owner:LKC TECH
Methods
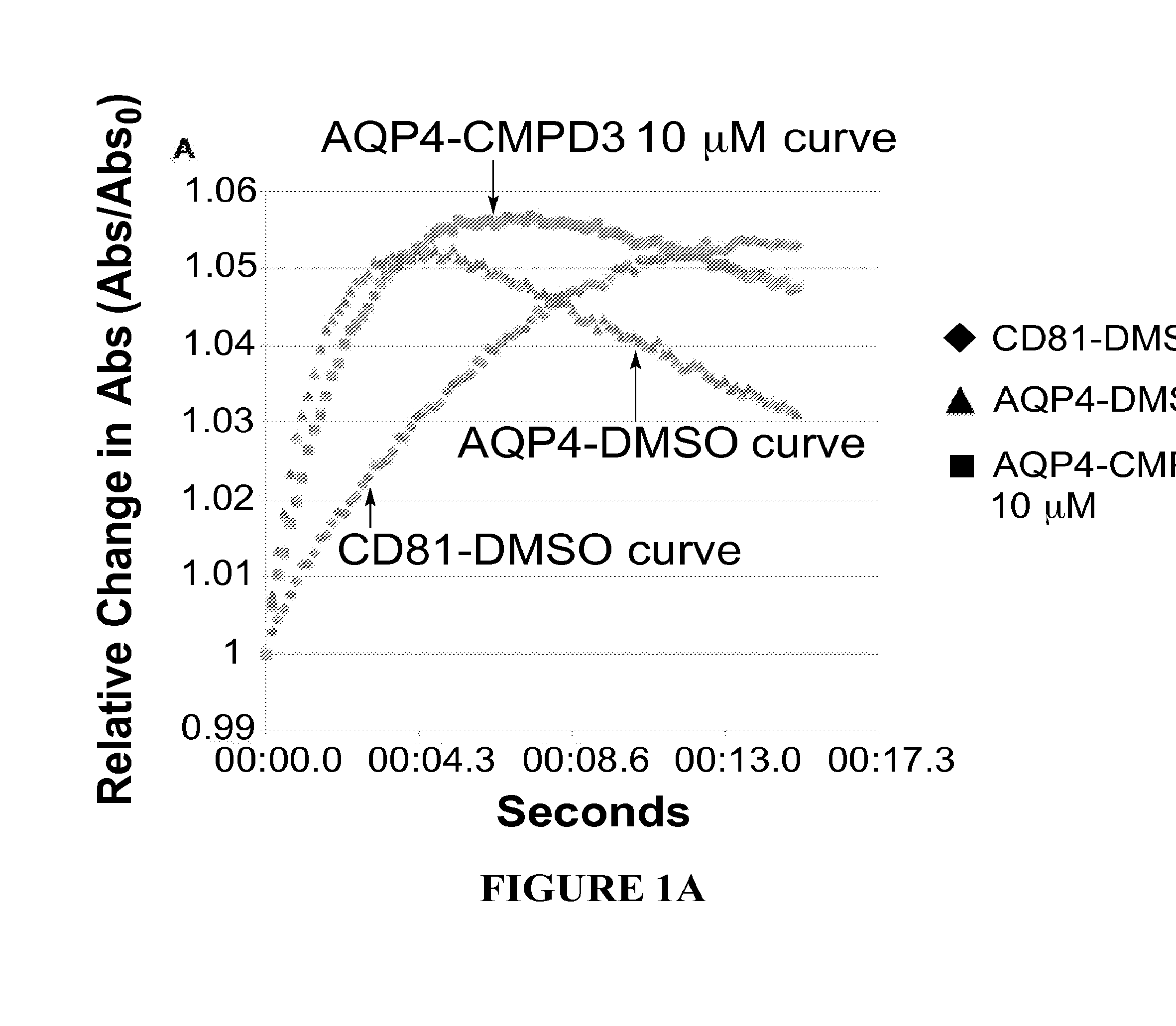
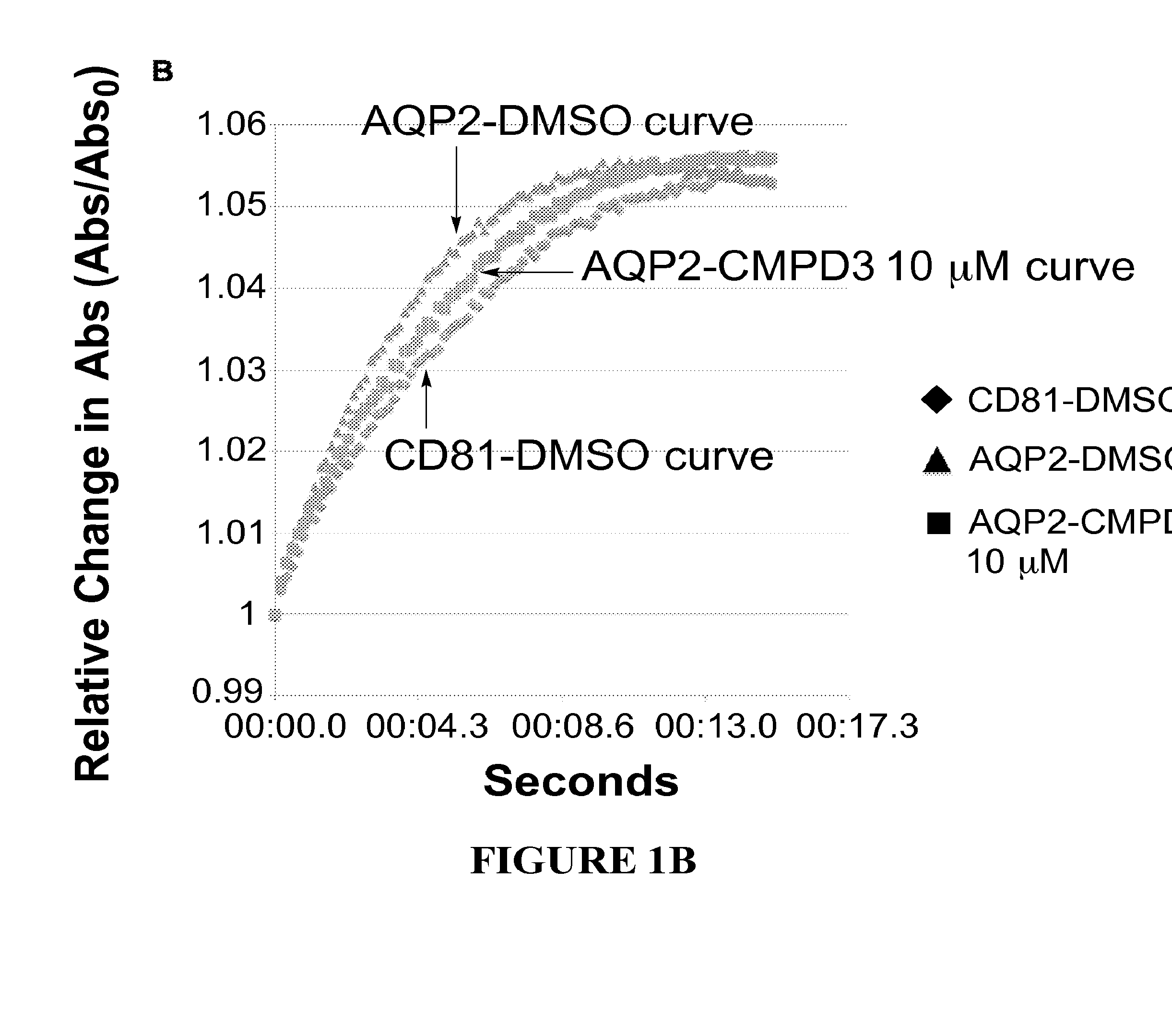
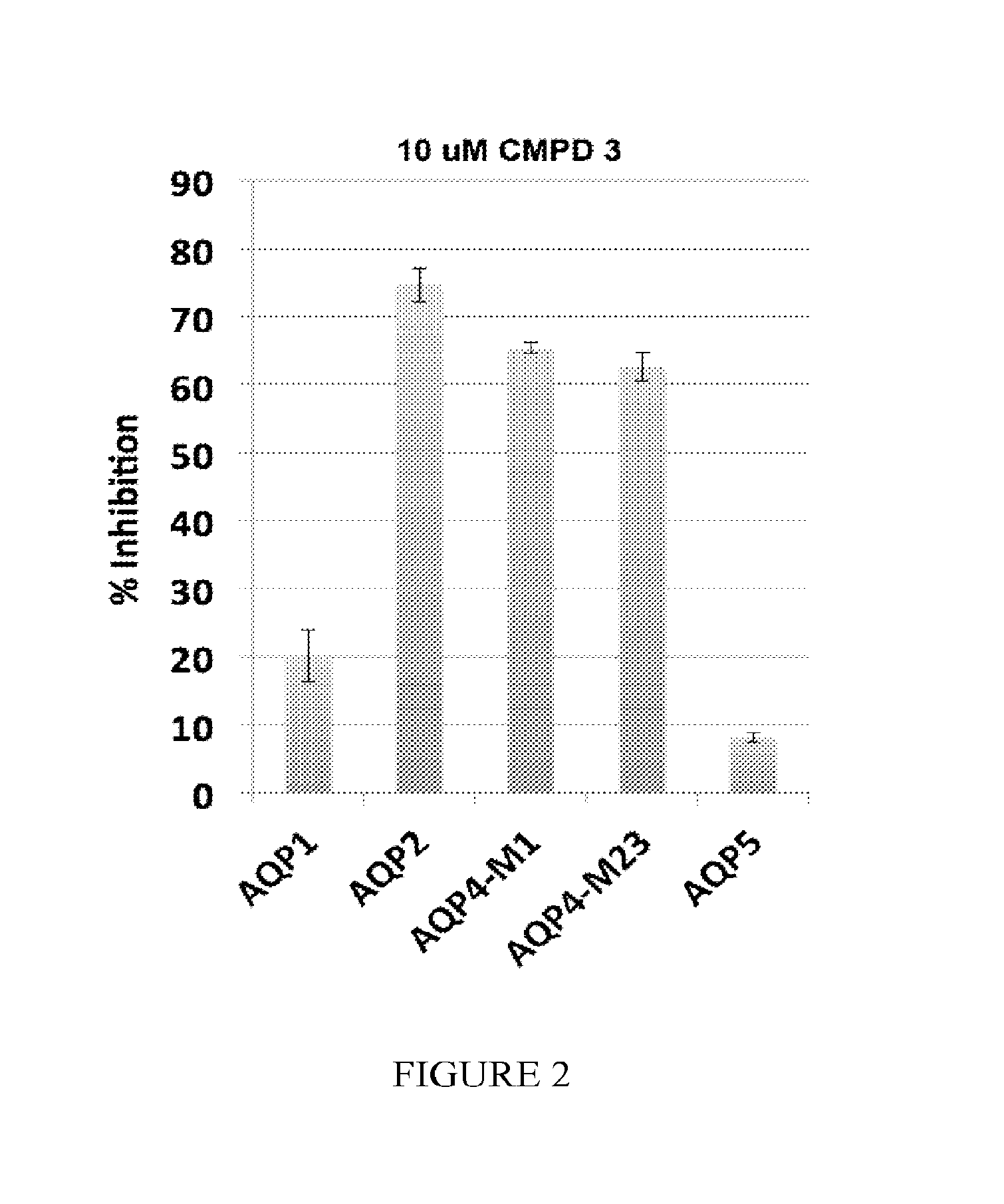
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
Non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy and the treatment of other ocular conditions
InactiveUS20060257391A1Prevent and reduce rateReduce inflammationSenses disorderPeptide/protein ingredientsSerine proteinasesDisease
A non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy to the proliferative form of diabetic retinopathy comprising intravitreally administering to a patient suffering from non-proliferative diabetic retinopathy an effective amount of serine proteinase enzyme sufficient to create, without surgery, a posterior vitreal detachment to prevent or reduce the progression of proliferative diabetic retinopathy in said patient. Also disclosed is a non-surgical method of treating ocular conditions such as retinal ischemia, retinal inflammation, retinal edema tractional retinal detachment, tractional retinopathy, vitreous hemorrhage and tractional maculopathy by intravitreally administering to a patient suffering from one or more of these conditions with an effective amount of a serine proteinase enzyme to reduce or treat that particular ocular condition. Plasmin, microplasmin and miniplasmin are preferred serine proteinase enzymes and plasmin is the most preferred.
Owner:BAUSCH & LOMB INC
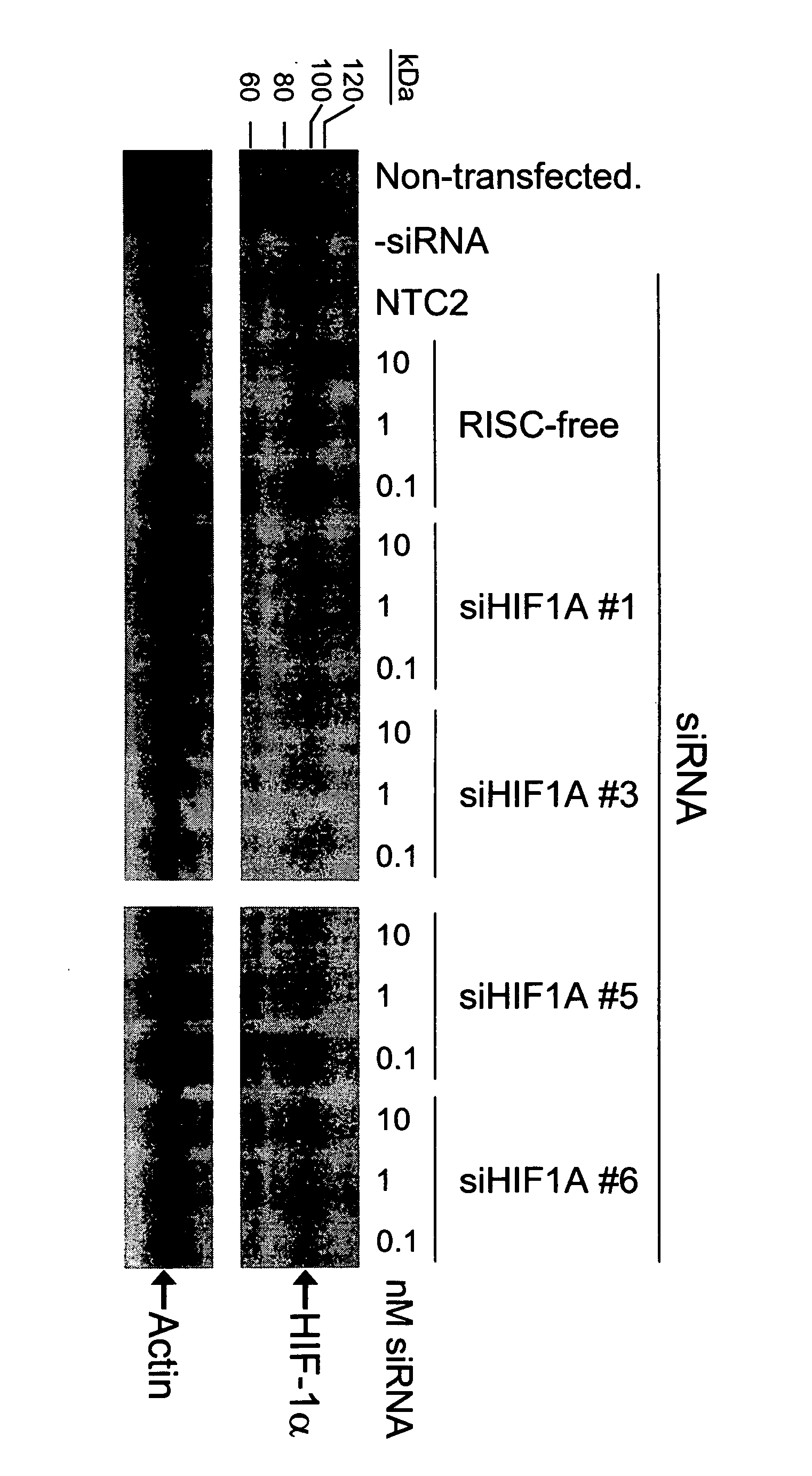
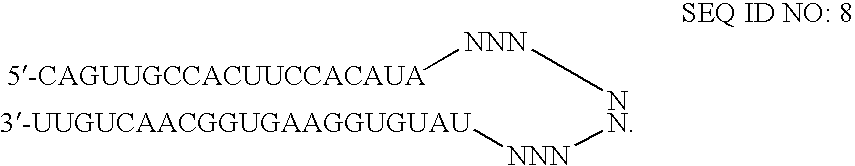
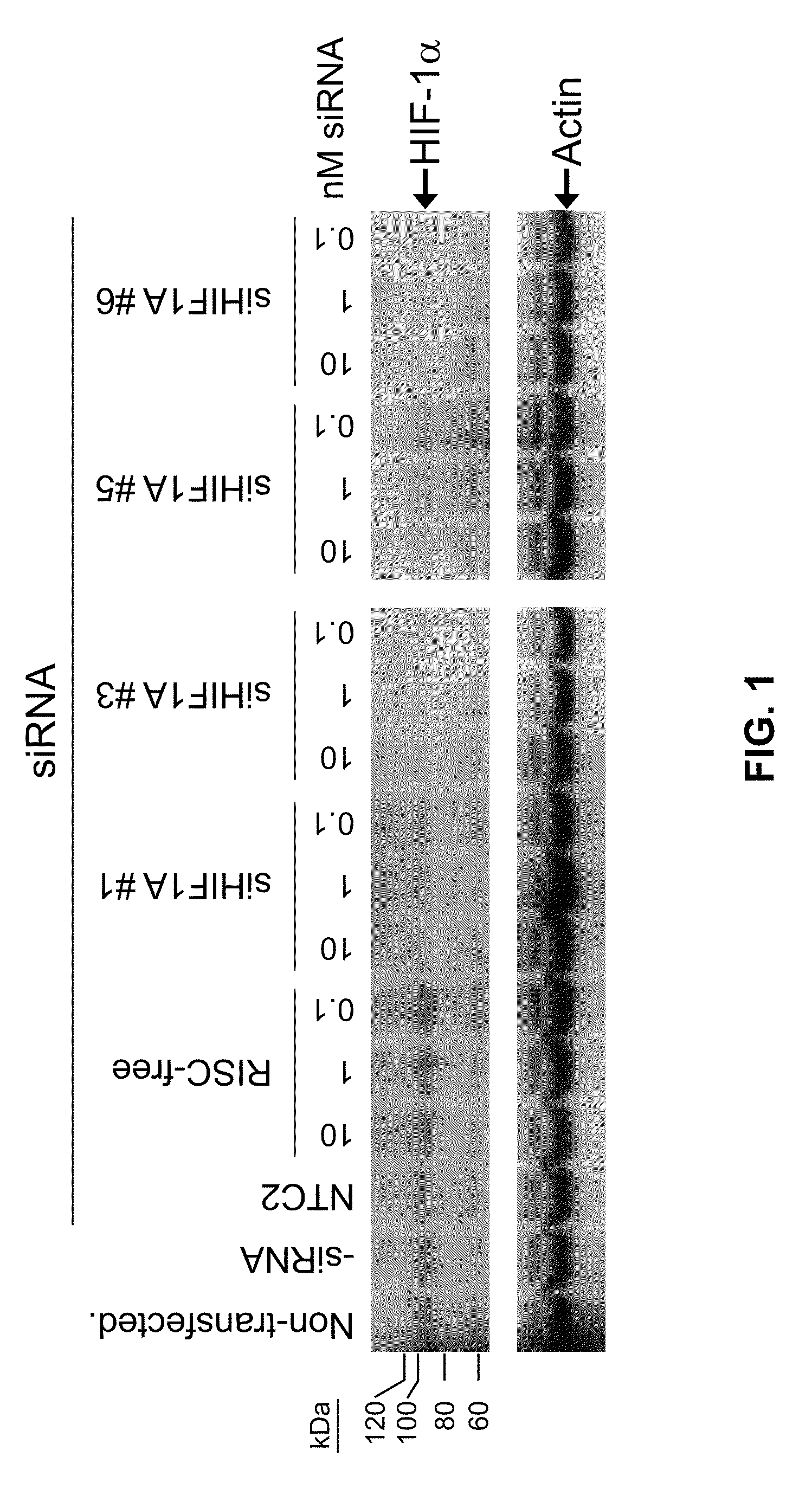
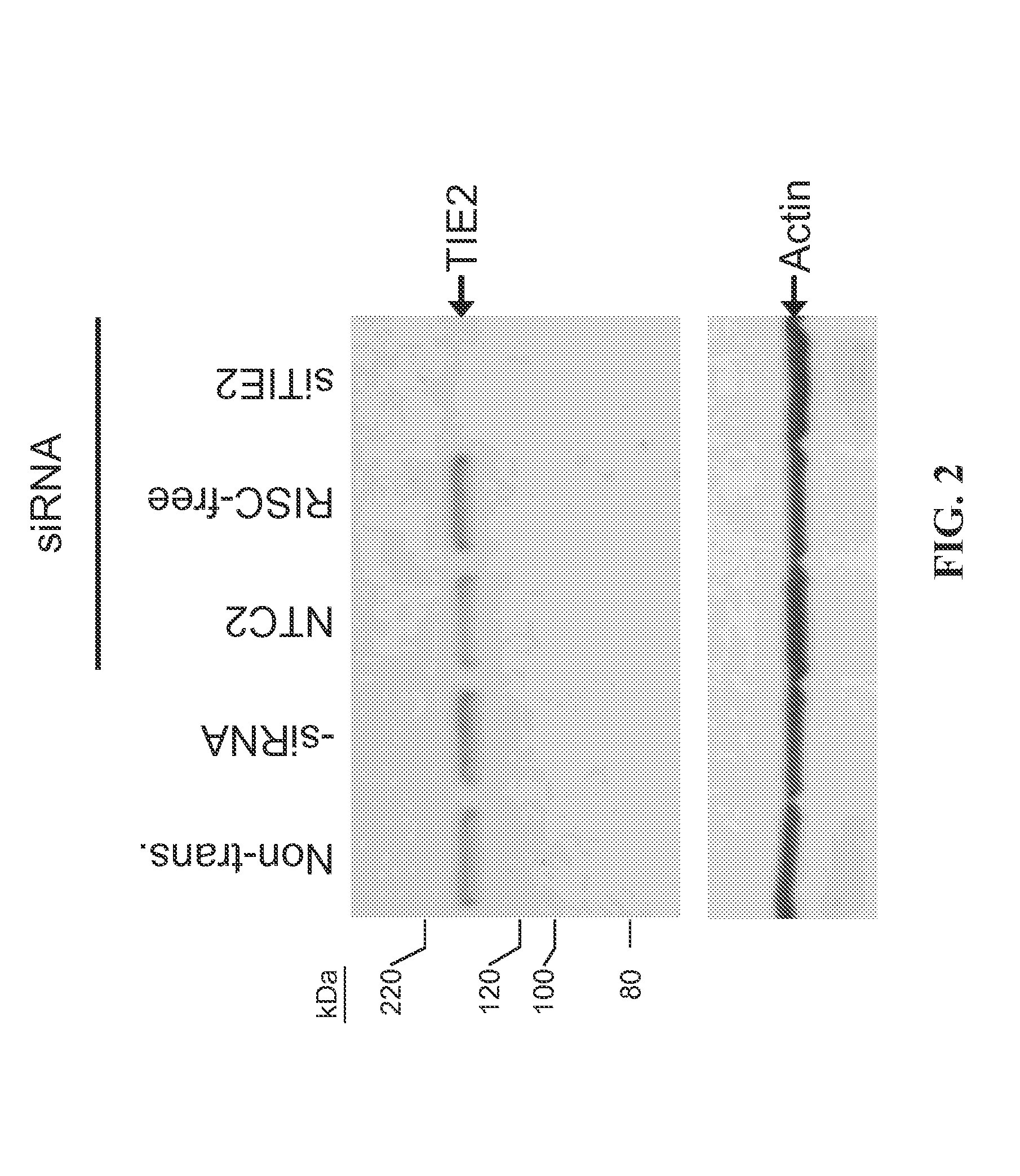
RNAi-mediated inhibition of HIF1A for treatment of ocular angiogenesis
InactiveUS20070155690A1Decreasing transcriptional activityLowering of ocular pre-angiogenicOrganic active ingredientsSenses disorderSequelaHIF1A
RNA interference, is provided for inhibition of HIF1A mRNA expression for treating patients with ocular angiogenesis, particularly for treating retinal edema, diabetic retinopathy, sequela associated with retinal ischemia, posterior segment neovascularization (PSNV), and neovascular glaucoma, and for treating patients at risk of developing such conditions.
Owner:ARROWHEAD RES CORP
Non-Surgical Method for Preventing or Reducing the Rate of the Progression of Non-Proliferative Diabetic Retinopathy and the Treatment of Other Ocular Conditions
InactiveUS20070128182A1Reduce inflammationSenses disorderPeptide/protein ingredientsDiseaseSerine proteinases
A non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy to the proliferative form of diabetic retinopathy comprising intravitreally administering to a patient suffering from non-proliferative diabetic retinopathy an effective amount of serine proteinase enzyme sufficient to create, without surgery, a posterior vitreal detachment to prevent or reduce the progression of proliferative diabetic retinopathy in said patient. Also disclosed is a non-surgical method of treating ocular conditions such as retinal ischemia, retinal inflammation, retinal edema tractional retinal detachment, tractional retinopathy, vitreous hemorrhage and tractional maculopathy by intravitreally administering to a patient suffering from one or more of these conditions with an effective amount of a serine proteinase enzyme to reduce or treat that particular ocular condition. Plasmin, microplasmin and miniplasmin are preferred serine proteinase enzymes and plasmin is the most preferred.
Owner:BARTELS STEPHEN P +3
Compositions and methods for treatment of ophthalmic diseases and disorders
InactiveUS20090197967A1Prevent degradationSlow chromophore fluxBiocideSenses disorderDiseaseDiabetic maculopathy
Provided herein are compositions and methods for treating ophthalmic diseases and disorders. Compositions comprising retinylamine derivative compounds provided herein are useful for treating and preventing ophthalmic diseases and disorders, including diabetic retinopathy diabetic maculopathy, diabetic macular edema, retinal ischemia, ischemia-reperfusion related retinal injury, and metabolic optic neuropathy.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION +1
Device to monitor retinal ischemia
A battery powered, hand held device to determine of there is significant retinal ischemia in the eye of a patient. The device detects the consequences of impaired blood flow in the eye of the patient and has a light source for emitting a light and a diffuser or diffuse spheroidal reflector that redirects the light from the light source toward the patient's eye. A set of electrodes contact the patients skin proximate to the eye and receives an electrical signal representing the eye's electrical response to the light stimulus. A microcontroller interprets the electrical signal sensed by the electrodes by using an algorithm to determine the degree of retinal ischemia of the patient. In one embodiment, there is a control that establishes the intensity of the light stimulus by measuring and using the area of the pupil.
Owner:LKC TECH
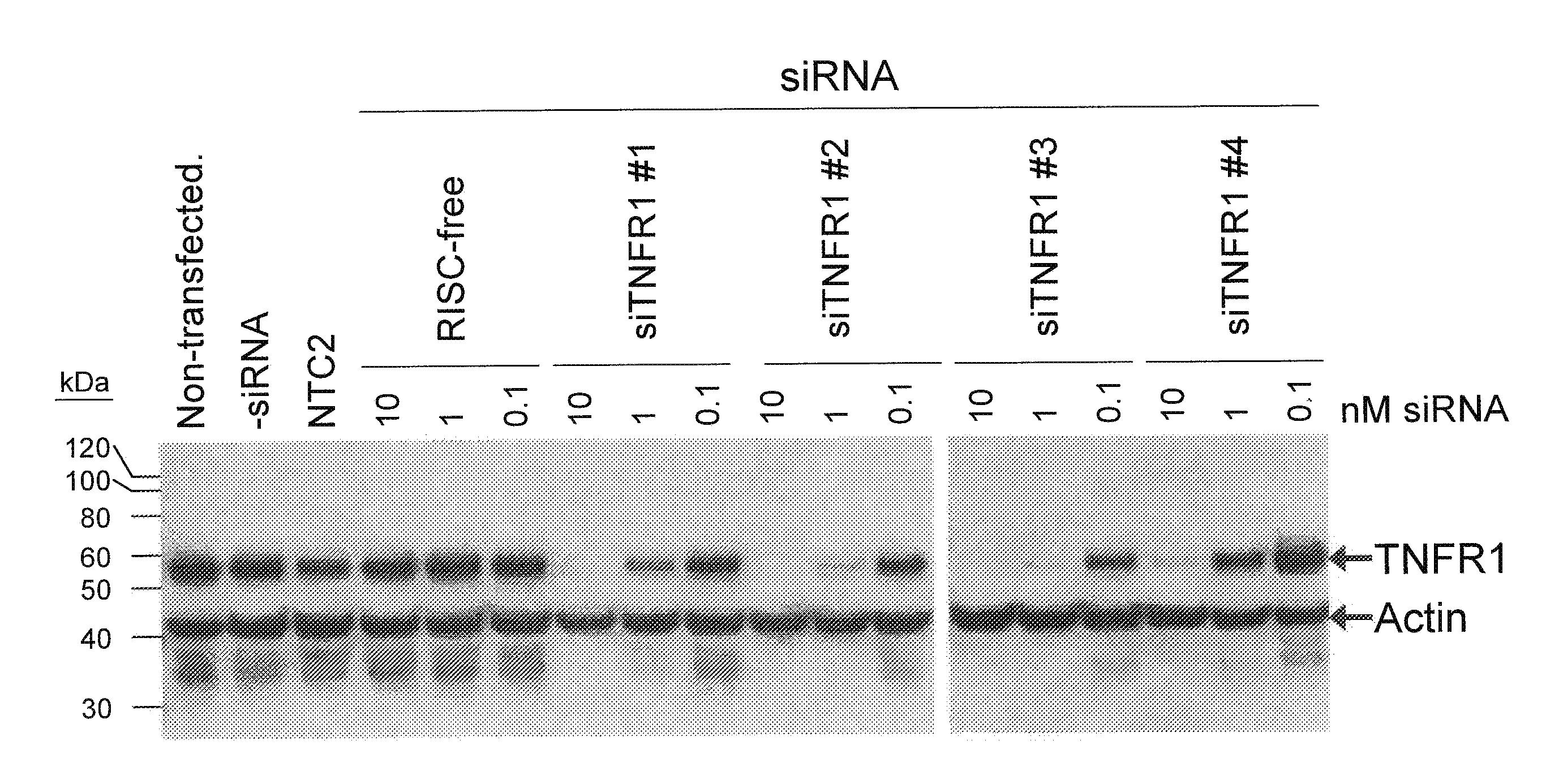
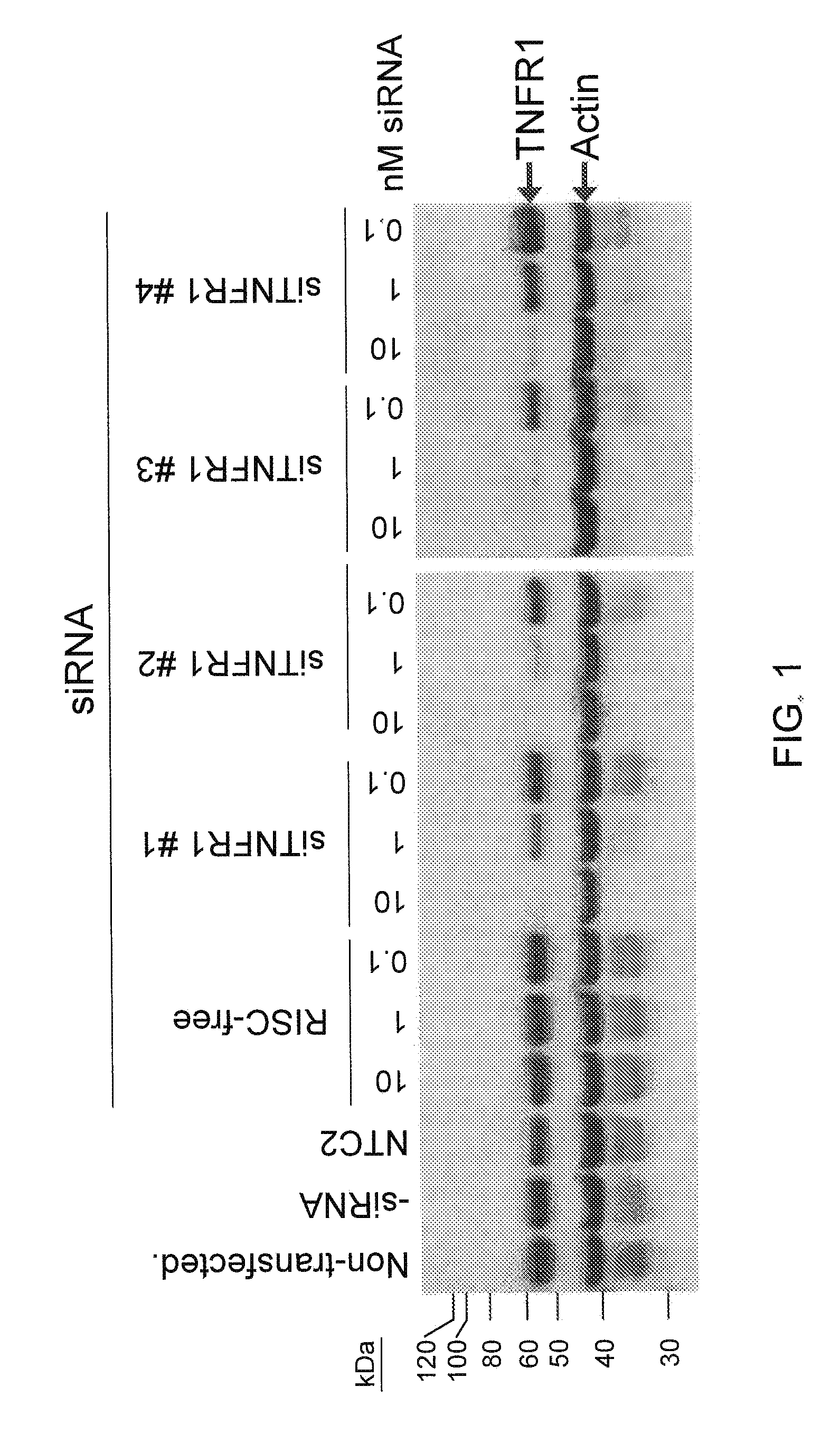
RNAi-RELATED INHIBITION OF TNFalpha SIGNALING PATHWAY FOR TREATMENT OF OCULAR ANGIOGENESIS
InactiveUS20090036396A1Organic active ingredientsSenses disorderReceptor degradationTnfα converting enzyme
RNA interference is provided for inhibition of tumor necrosis factor α (TNFα) by silencing TNFα cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFα converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFα targets, in particular, is useful for treating patients having a TNFα-related condition or at risk of developing a TNFα-related condition, such as ocular angiogenesis, retinal ischemia, and diabetic retinopathy.
Owner:ARROWHEAD RES CORP
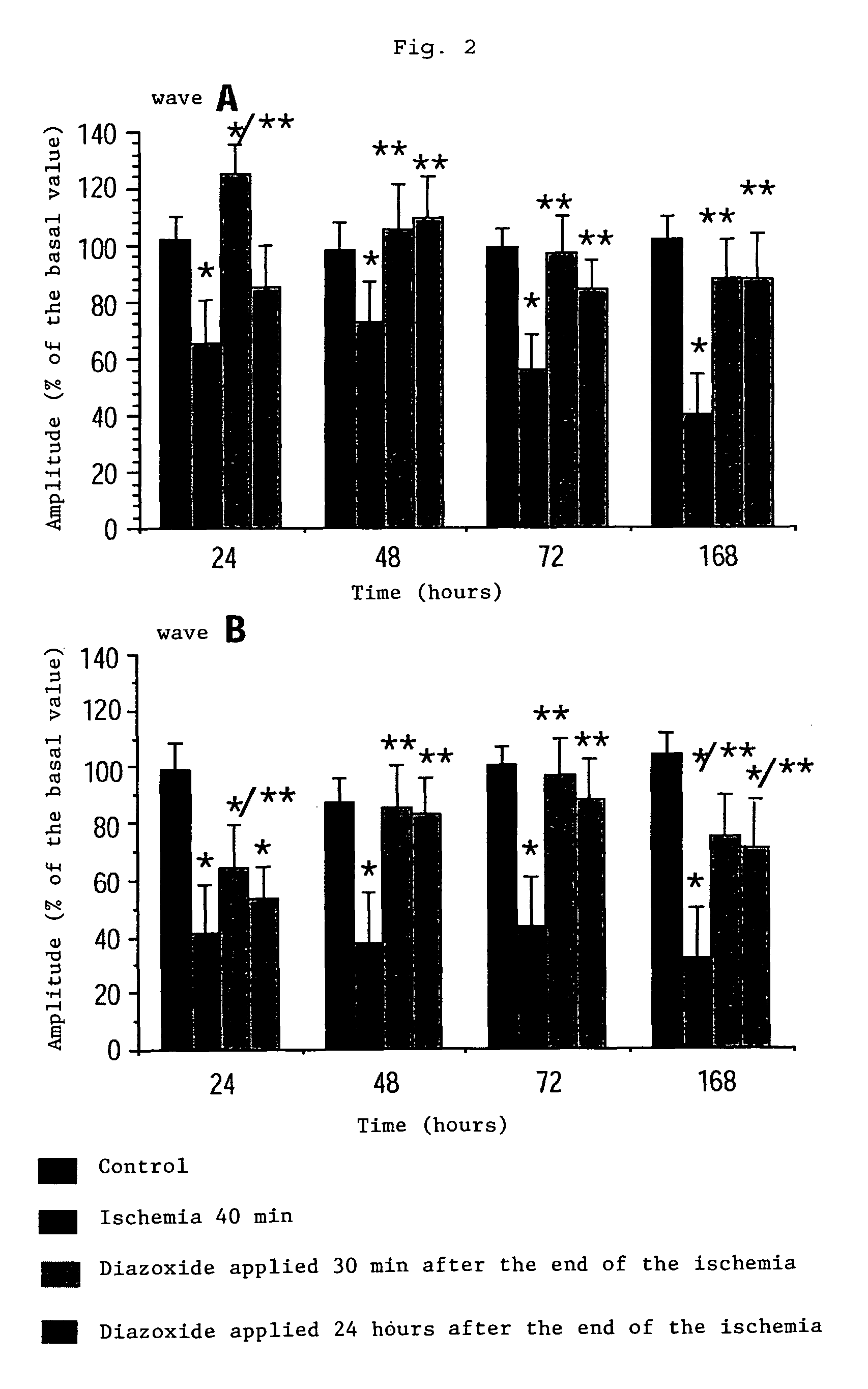
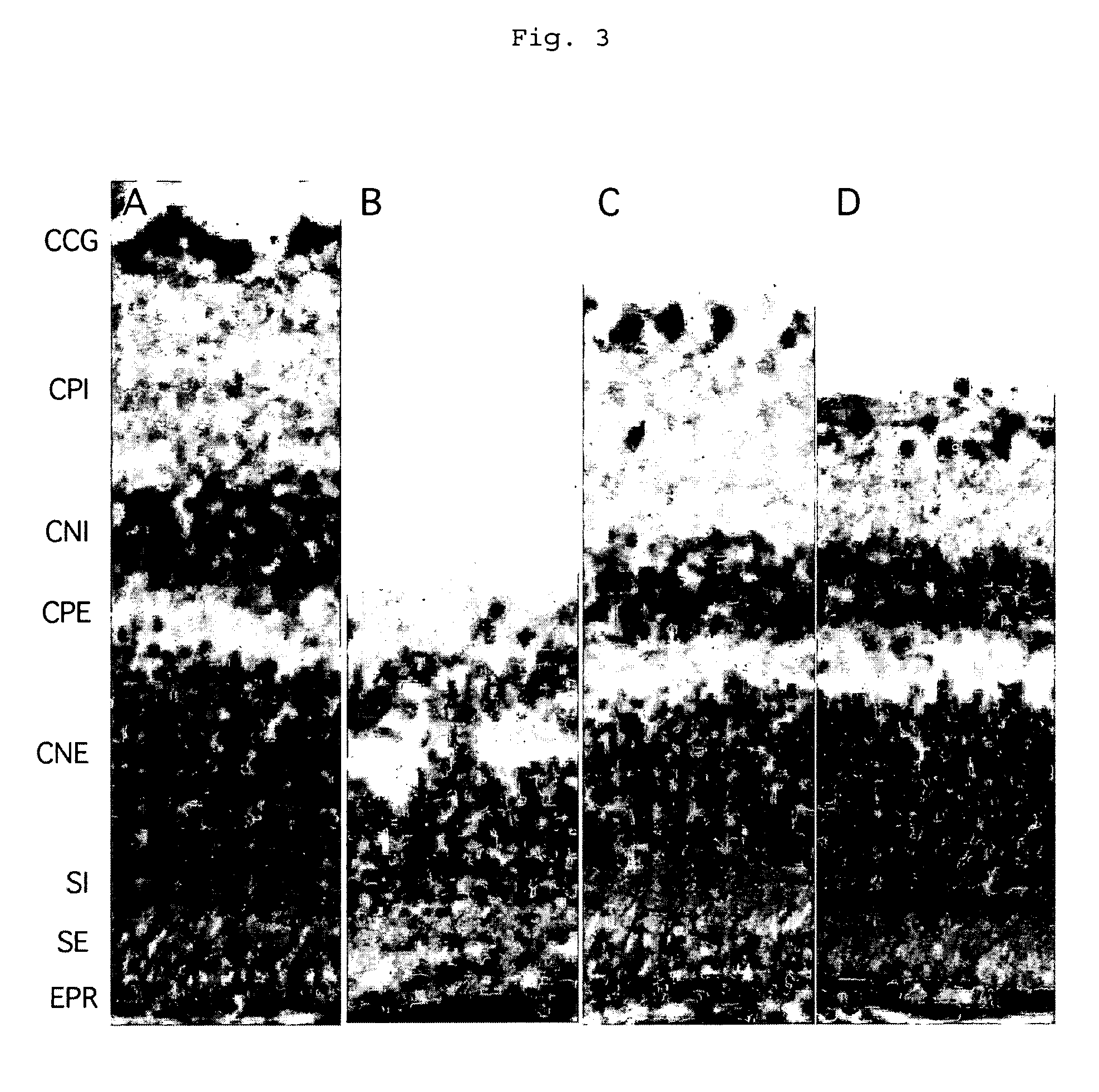
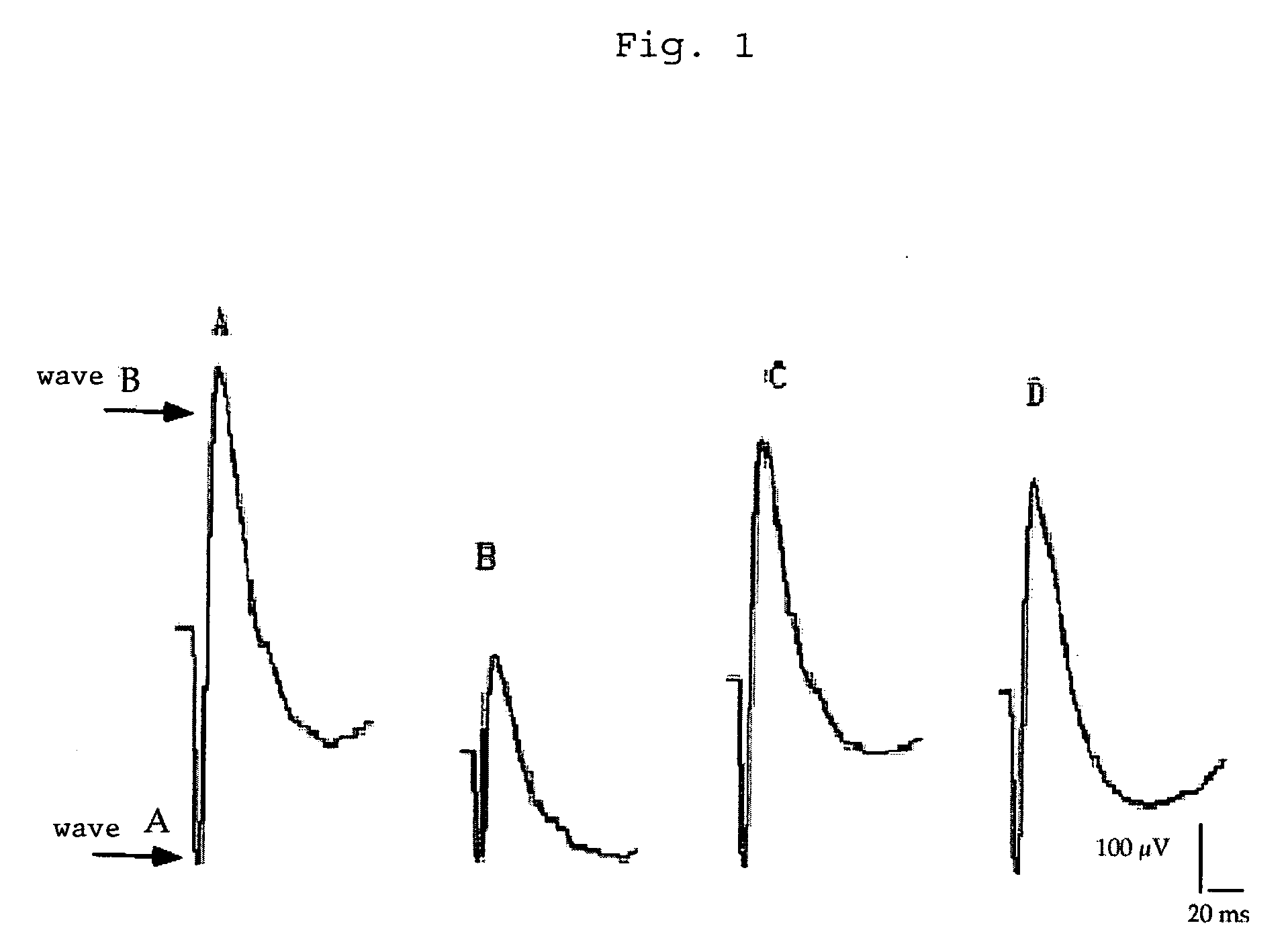
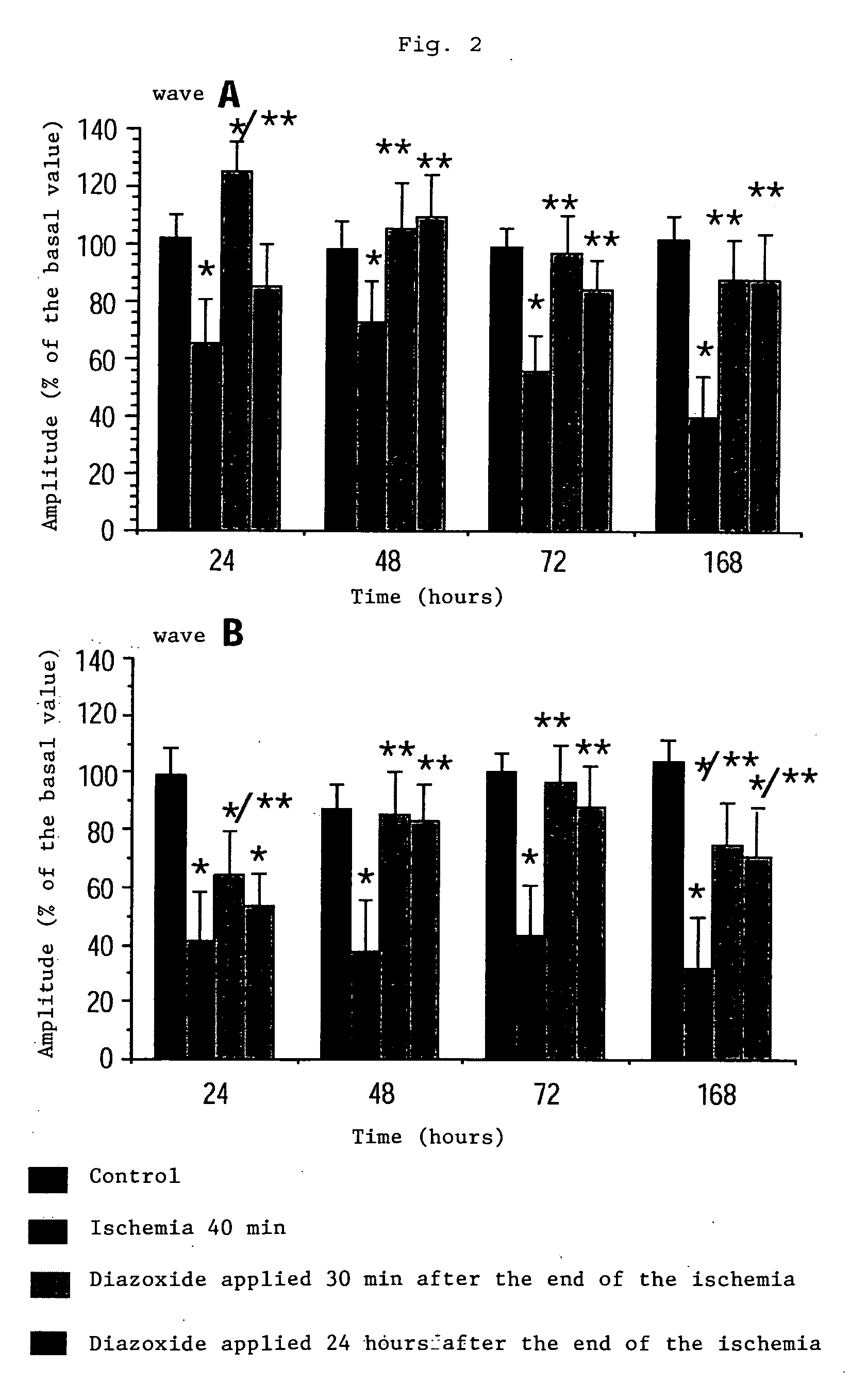
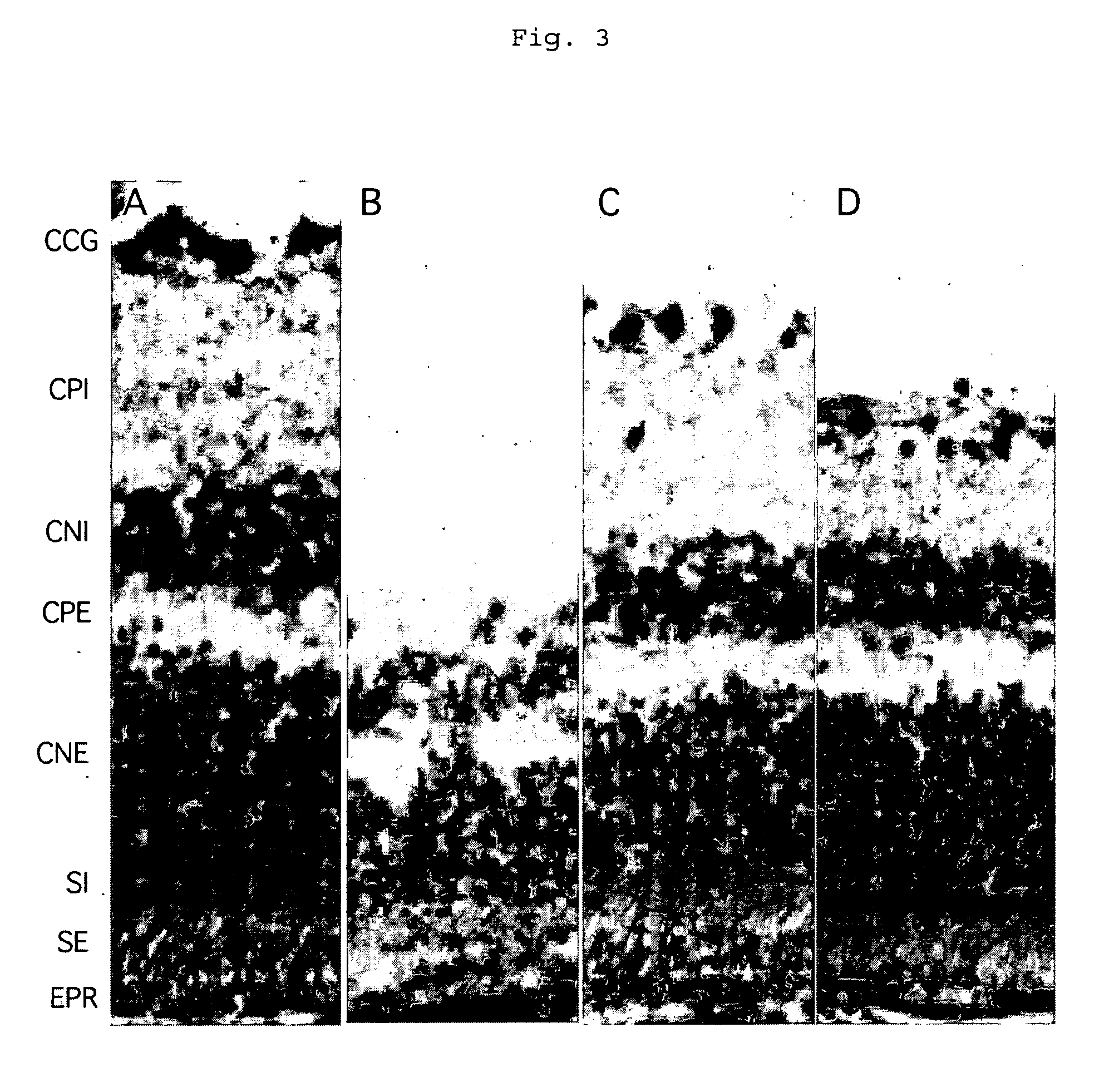
Method of treatment of retinal ischemia with diazoxide
A composition including diazoxide (7-chloro-3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxide) for the treatment and / or prevention of retinal ischemia and of diseases associated with retinal ischemia. The composition can also contain riluzole, a derivative active in neuroprotection of the latter, or a pharmaceutically acceptable salt of the latter.
Owner:CENT NAT DE LA RECHERCHE SCI +1
RNAi-MEDIATED INHIBITION OF H1F1A FOR TREATMENT OF OCULAR ANGIOGENESIS
RNA interference is provided for inhibition of HIF1A mRNA expression for treating patients with ocular angiogenesis, particularly for treating retinal edema, diabetic retinopathy, sequela associated with retinal ischemia, posterior segment neovascularization (PSNV), and neovascular glaucoma, and for treating patients at risk of developing such conditions.
Owner:ARROWHEAD RES CORP
Tyrosine kinase inhibitors
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Method of treatment of retinal ischemia with diazoxide
A composition including diazoxide (7-chloro-3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxide) for the treatment and / or prevention of retinal ischemia and of diseases associated with retinal ischemia. The composition can also contain riluzole, a derivative active in neuroprotection of the latter, or a pharmaceutically acceptable salt of the latter.
Owner:CENT NAT DE LA RECHERCHE SCI +1
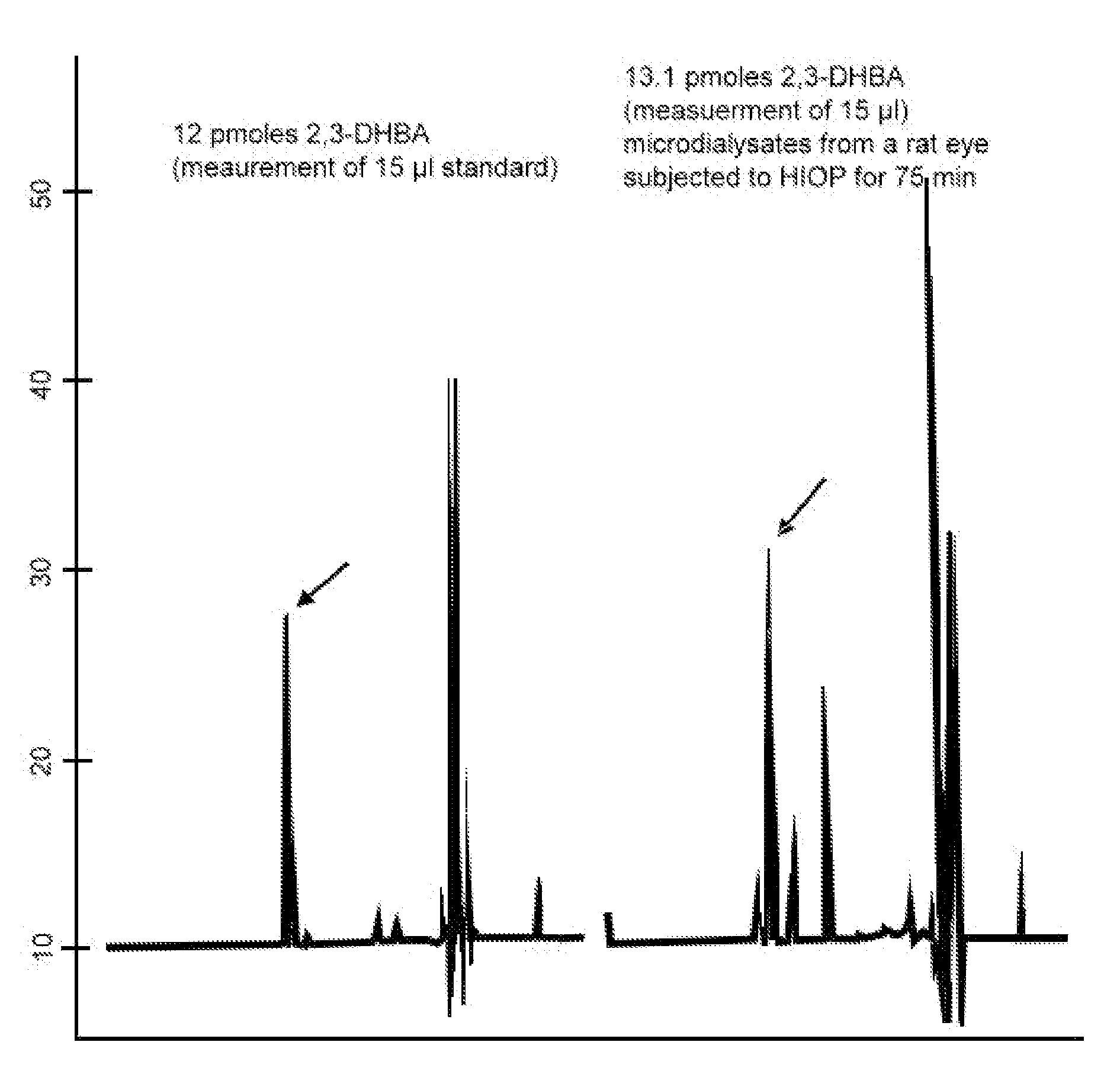
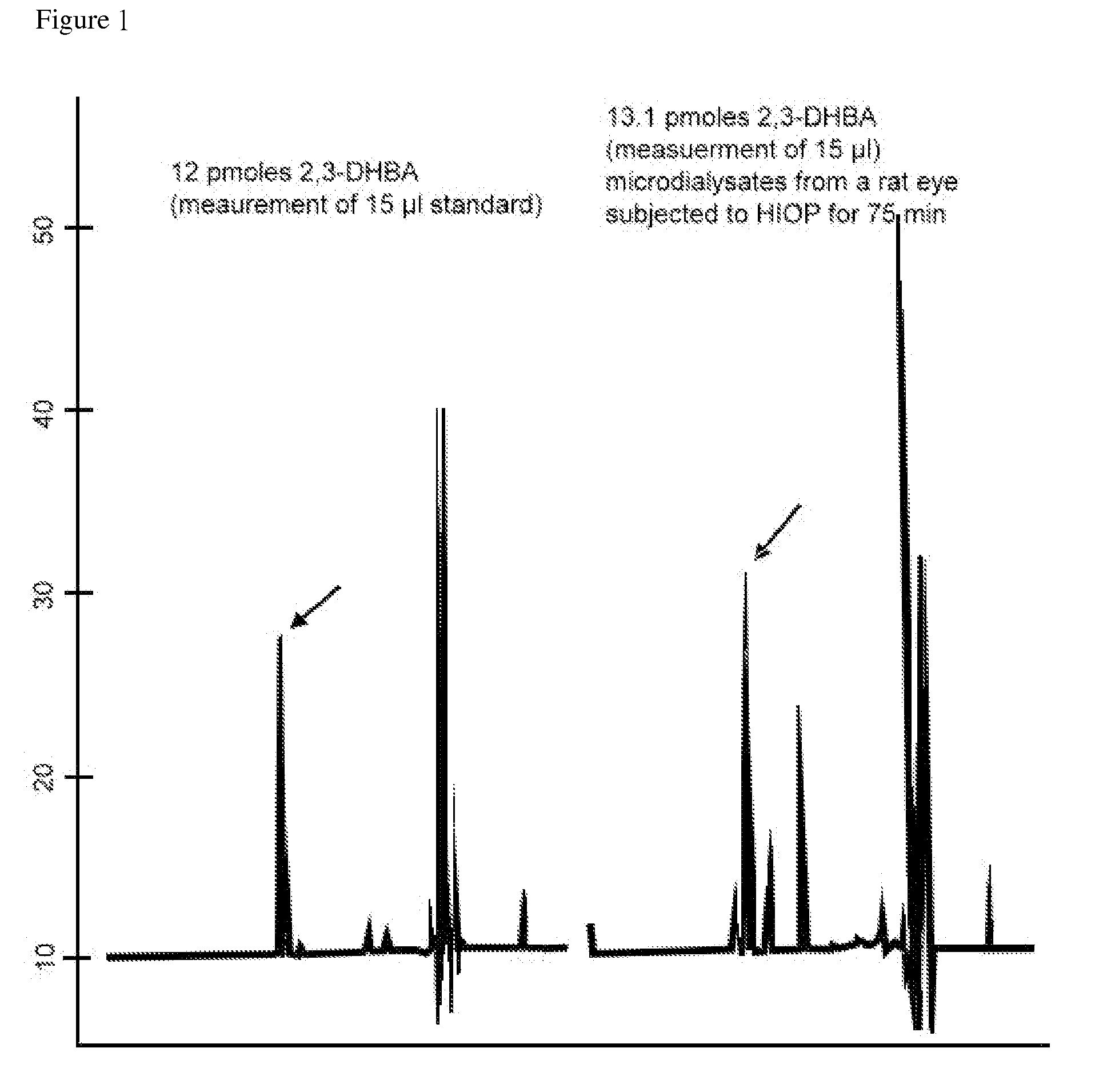
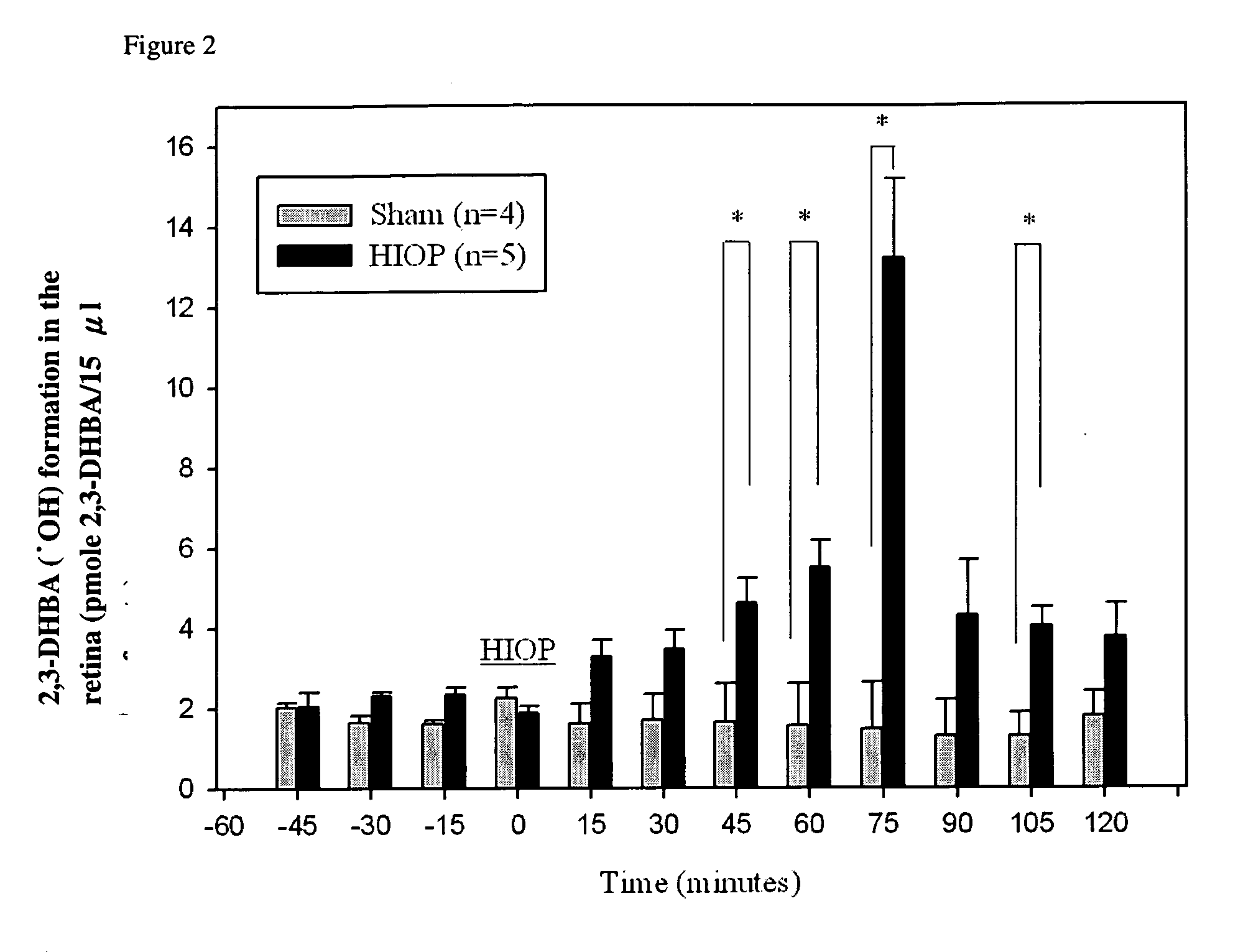
Protection of ferulic acid and/or tetramethylpyrazine against retinal ischaemia, glaucoma and siderosis oculi
A method for preventing and / or treating ischaemic and / or iron-related retinal or brain disorders comprising administering an effective amount of ferulic acid (FA), tetramethylpyrazine (TMP) or their pharmaceutically acceptable salt, ester, solvate, hydrate, analogs, metabolite, enantiomer, isomer, tautomer, amide, derivative or prodrug to a subject. The former diseases comprise retinal ischemia, glaucoma as well as brain ischaemia (i.e. stroke, infarction typed). The latter ones comprise age-related macular degeneration, intraocular hemorrhage, siderosis oculi (due to retained intraocular iron), oxidative stress of the retina as well as brain hemorrhage (stroke, hemorrhagic type) or Alzheimer disease. Clinically, FA alone or in combination of TMP can be administered systemically, orally, intravitreously, topically (in form of eyedrops), as well as other routes such as periocular, subconjunctival, and intracamera.
Owner:COMMITTEE ON CHINESE MEDICINE & PHARMACY DEPT OF HEALTH EXECUTIVE YUAN
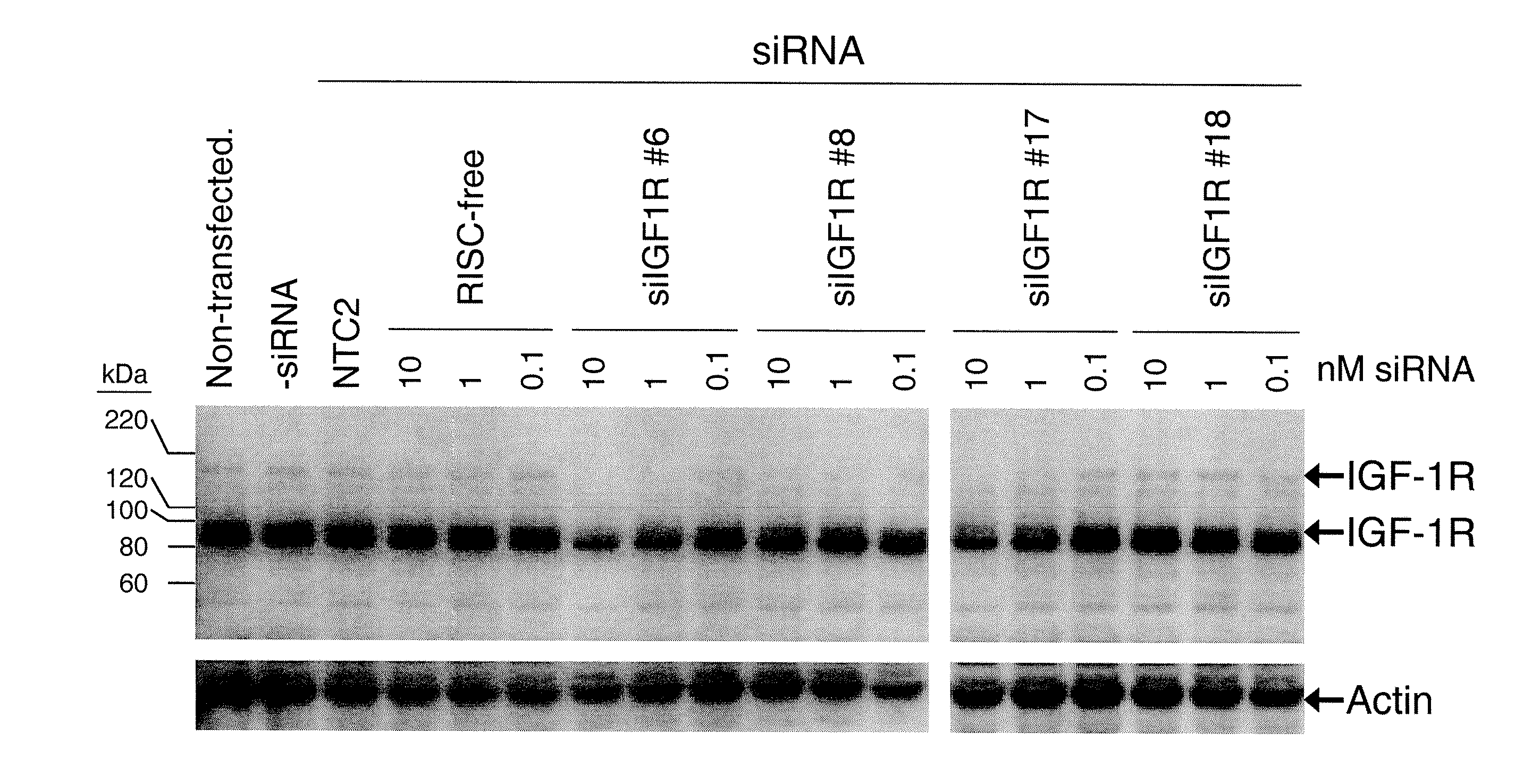
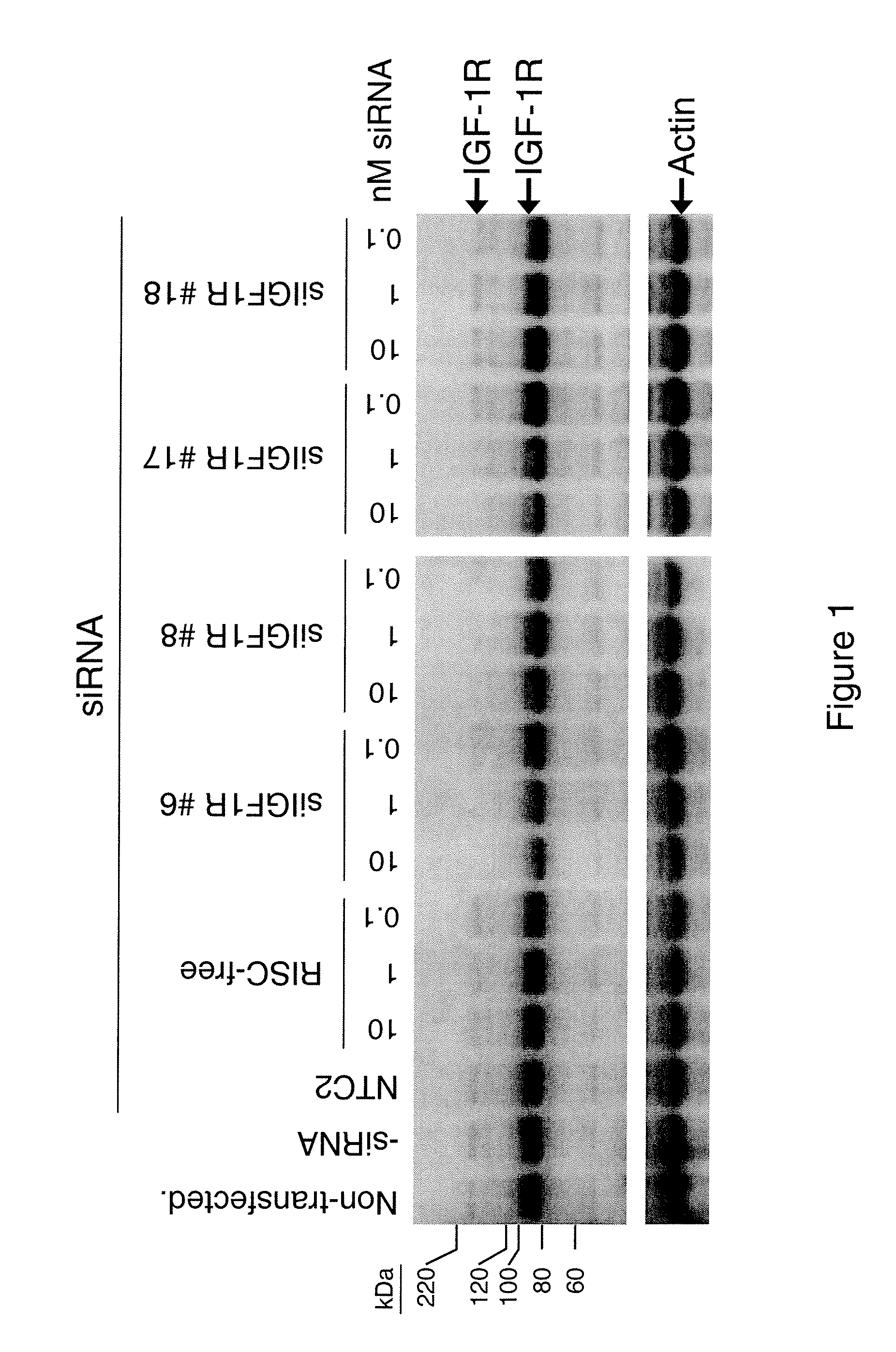
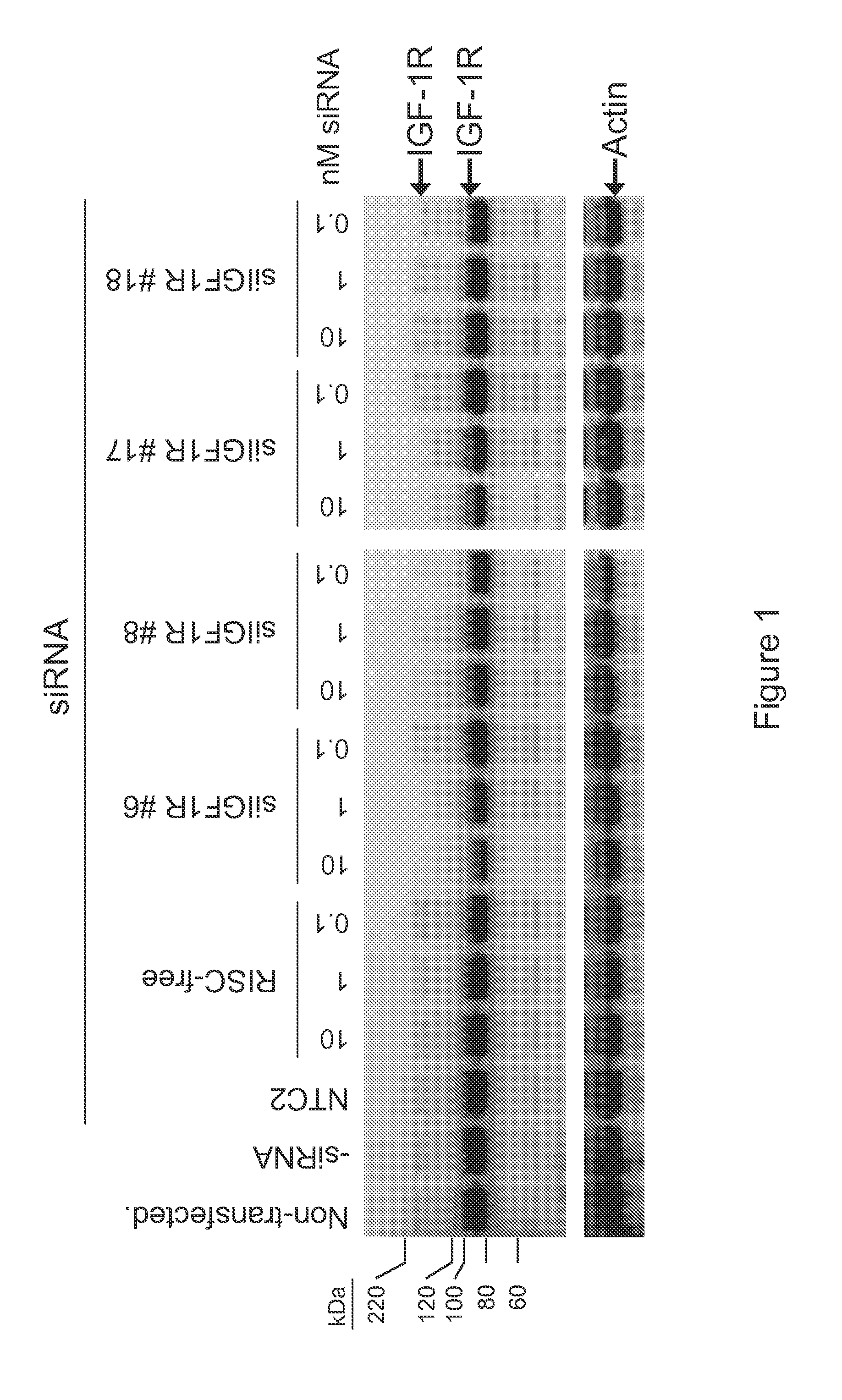
RNAi-MEDIATED INHIBITION OF IGF1R FOR TREATMENT OF OCULAR ANGIOGENESIS
InactiveUS20070275922A1Reduced activityLowering of ocular pre-angiogenicOrganic active ingredientsSenses disorderSequelaOcular angiogenesis
RNA interference is provided for inhibition of IGF1R mRNA expression for treating patients with ocular angiogenesis, particularly for treating retinal edema, diabetic retinopathy, sequela associated with retinal ischemia, posterior segment neovascularization (PSNV), and neovascular glaucoma, and for treating patients at risk of developing such conditions.
Owner:ARROWHEAD RES CORP
Tyrosine kinase inhibitors
InactiveUS20060183755A1BiocideOrganic active ingredientsDiabetic retinopathyTyrosine-kinase inhibitor
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
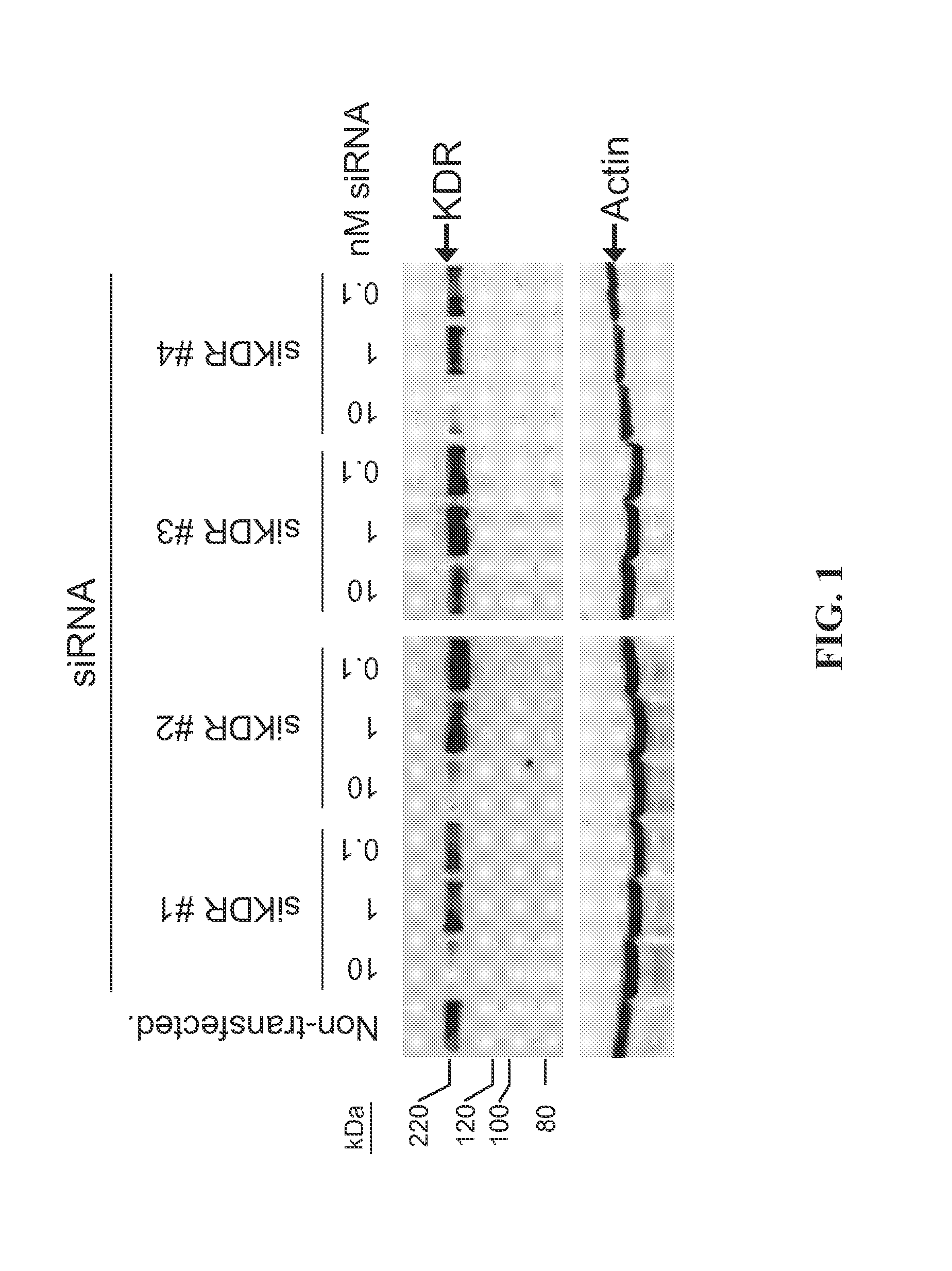
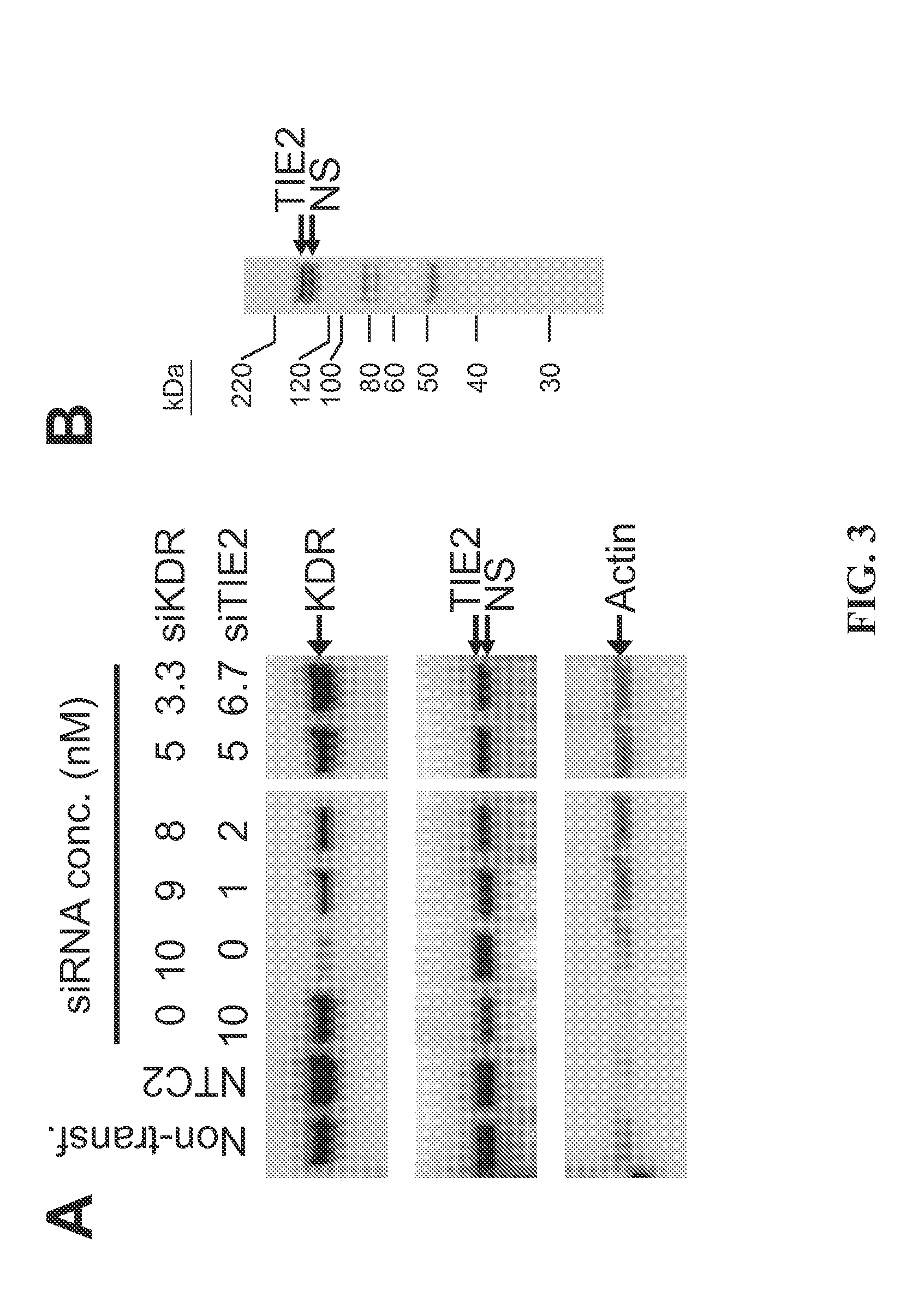
RNAi-MEDIATED INHIBITION OF SELECT RECEPTOR TYROSINE KINASES FOR TREATMENT OF PATHOLOGIC OCULAR NEOVASCULARIZATION-RELATED CONDITIONS
InactiveUS20140200259A1Reducing signal transductionLowering of ocular pre-angiogenicFermentationPlant genotype modificationDiseaseOcular neovascularization
RNA interference is provided for inhibiton of expression of select receptor tyrosine kinase (RTK) targets in ocular neovascularization-related conditions, including those cellular changes resulting from the signal transduction activity of the select RTK targets that lead directly or indirectly to ocular NV, abnormal angiogenesis, retinal vascular permeability, retinal edema, diabetic retinopathy particularly proliferative diabetic retinopathy, diabetic macular edema, exudative age-related macular degeneration, sequela associated with retinal ischemia, and posterior segment neovascularization.
Owner:NOVARTIS AG
Traditional Chinese medicine preparation for treating central retinal vein occlusion
InactiveCN103908627AImprove microcirculationImprove ischemia reperfusionSenses disorderCardiovascular disorderSalvia miltiorrhizaSide effect
A traditional Chinese medicine preparation is prepared from the following raw materials by weight: 25-30 g of prepared rehmannia root, 15-20 g of angelica sinensis, 10-15 g of radix paeoniae rubra, 8-10 g of rhizoma chuanxiong, 12-18 g of radix bupleuri, 15-20 g of platycodon grandiflorum, 15-20 g of fructus aurantii immaturus, 20-25 g of semen persicae, 15-20 g of radix curcumae, 12-18 g of salvia miltiorrhiza, 12-15 g of caulis spatholobi, 10-15 g of fructus lycii, and 6-10 g of licorice. The traditional Chinese medicine preparation is capable of regulating liver and promoting qi circulation, promoting blood circulation to remove blood stasis, and improving eyesight and dredging collaterals, and plays a role in treating both symptoms and root causes on the retinal vein occlusion. The traditional Chinese medicine preparation has the following clinical effects: 1, the traditional Chinese medicine preparation effectively treats the retinal vein occlusion, the clinical healing and conspicuous effect rate is 66.6%, and the total clinical effective rate is 93.3%; 2, the traditional Chinese medicine preparation can improve eyesight, improves retinal microcirculation and retinal ischemia reperfusion, and promotes retinal hemorrhage and exudation absorption, so as to achieve the purpose of treating the retinal vein occlusion; and 3, the traditional Chinese medicine preparation is convenient to take, and has no toxic or side effect.
Owner:杨丽敏
Medicinal composition for prevention or treatment retinal ischemia
This invention relates to a use of producing a medicinal composition for preventing or treating a disease, disorder or condition induced by retina ischemia, wherein the medicinal composition comprising 4.5 units by weight of Radix Angelicae Sinensis (Dang Gui), 4.5 units by weight of Radix Rehmanniae glutinosae (Di Huang), 3 units by weight of Radix Paeoniae Rubra (Chi Shao), from 2.25 to 2.3 units by weight of Rhizoma Ligustici Chuanxiong (Chuan Xiong), 6 units by weight of Semen Pruni Persicae (Táo Rén), 4.5 units by weight of Flos Carthami Tinctorii (Hong Hua), 1.5 units by weight of Radix Glycyrrhizae Uralensis (Gan Cao), 3 units by weight of Fructus Aurantii (Zhi Qiao), 1.5 units by weight of Radix Bupleuri Chinense (Chai Hu), 4.5 units by weight of Radix Achyranthis Bidentatae (Niu Xi), and from 2.25 to 2.3 units by weight of Radix Platycodi Grandiflori (Jie Geng).
Owner:CHAO FANG PING
Revascularization of ischemic retinal tissue and screening method therefor
The present invention provides a treatment for promoting beneficial physiological revascularization of ischemic retinal tissue. The treatment comprises administering to a mammal suffering from a retinal vascular ischemia a therapeutically effective amount of an angiostatic fragment of tryptophanyl-tRNA synthetase (TrpRS), thereby simultaneously inhibiting pathological neovascularization while promoting beneficial physiological revascularization of damaged areas of the retina. In a preferred embodiment, the angiostatic fragment of TrpRS is T2-TrpRS or T2-TrpRS-GD. Preferably, the mammal is a human patient suffering from retinal ischemia.
Owner:THE SCRIPPS RES INST
Prevention and treatment of retinal ischemia and edema
Owner:CHILDRENS MEDICAL CENT CORP
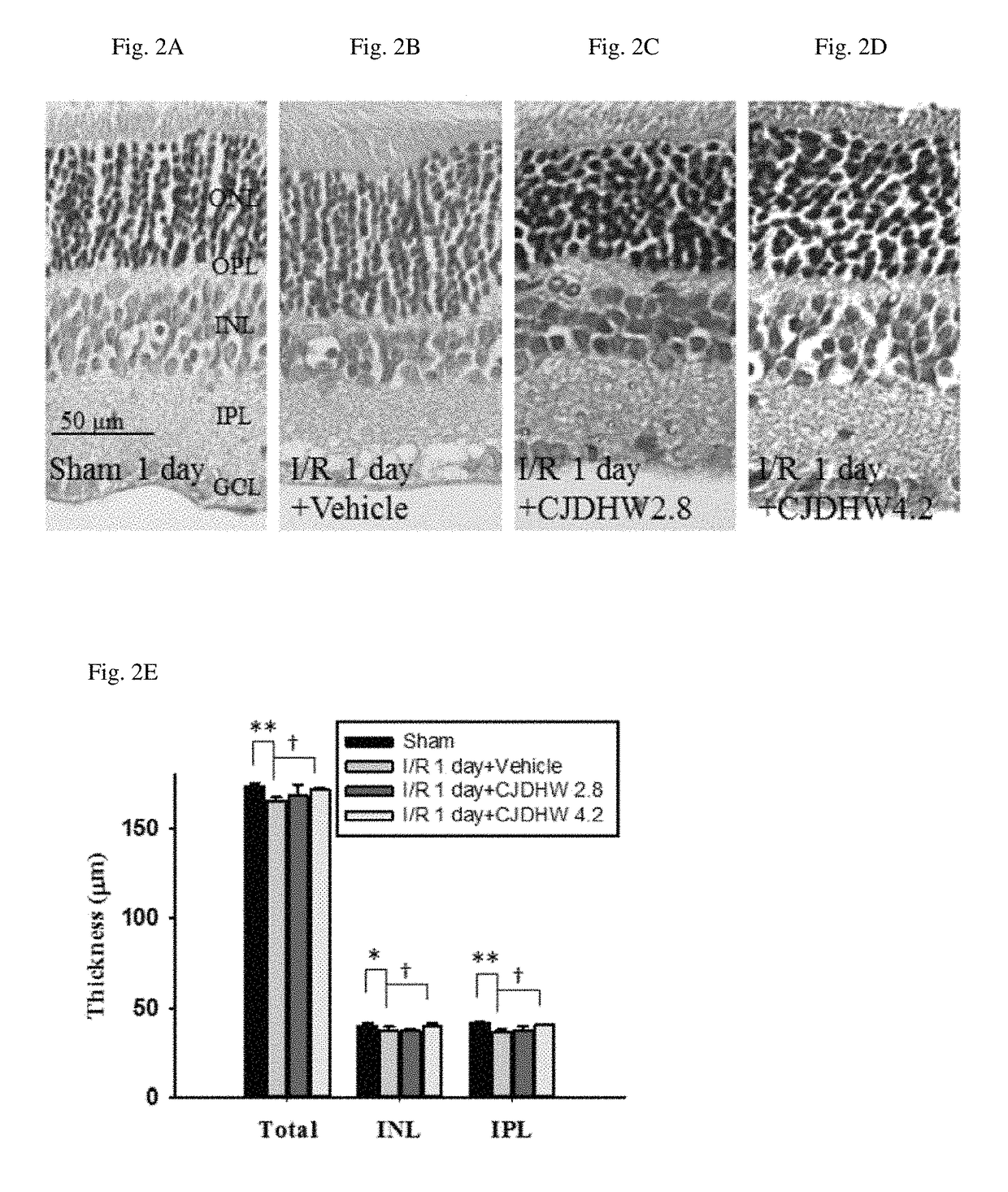
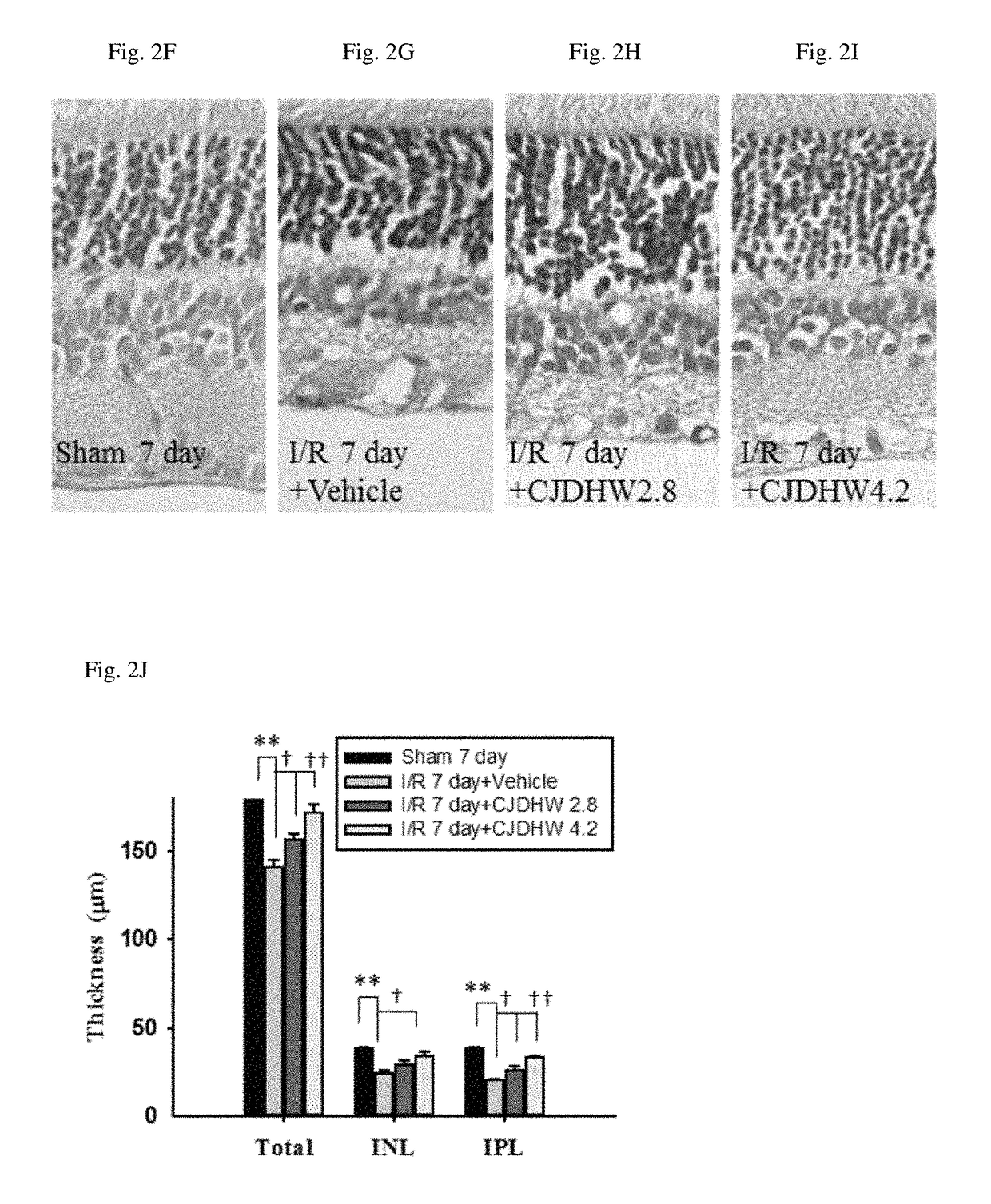
Use of chi-ju-di-huang-wan in treating retinal ischemia or a disease, condition, or disorder associated with retinal ischemia
The present invention relates to a method for treating retinal ischemia, or a disease, condition, or disorder associated with retinal ischemia, in a subject in need thereof, comprising administering to said subject a therapeutically effective amount of a composition comprising Chi-Ju-Di-Huang-Wan, wherein the Chi-Ju-Di-Huang-Wan consists of Rehmanniae Radix Preparata, Corni Fructus, Rhizoma Dioscoreae, Poria, Cortex Moutan Radicis, Alismatis Rhizome, Fructus Lycii, and Chrysanthemi Flos.
Owner:CHAO FANG PING
Use of hydroxycarbamide for preventing retinal nonperfusion
The present invention relates to the use of hydroxycarbamide (HC) for reducing and / or delaying the extension of capillary nonperfusion, a cause of irreparable visual impairment in patients suffering from central retinal vein occlusion (CRVO). This is the first systemic treatment which makes it possible to reduce retinal ischemic complications in patients in whom (CRVO) has been recently diagnosed and is consequently in a rapidly progressive phase. Given the low toxicity of HC evaluated on a large scale in children and adults in the context of other diseases for decades, the results of the present study open up a new therapeutic approach in the treatment of CRVO.
Owner:UNIVERSITÉ PARIS CITÉ +5
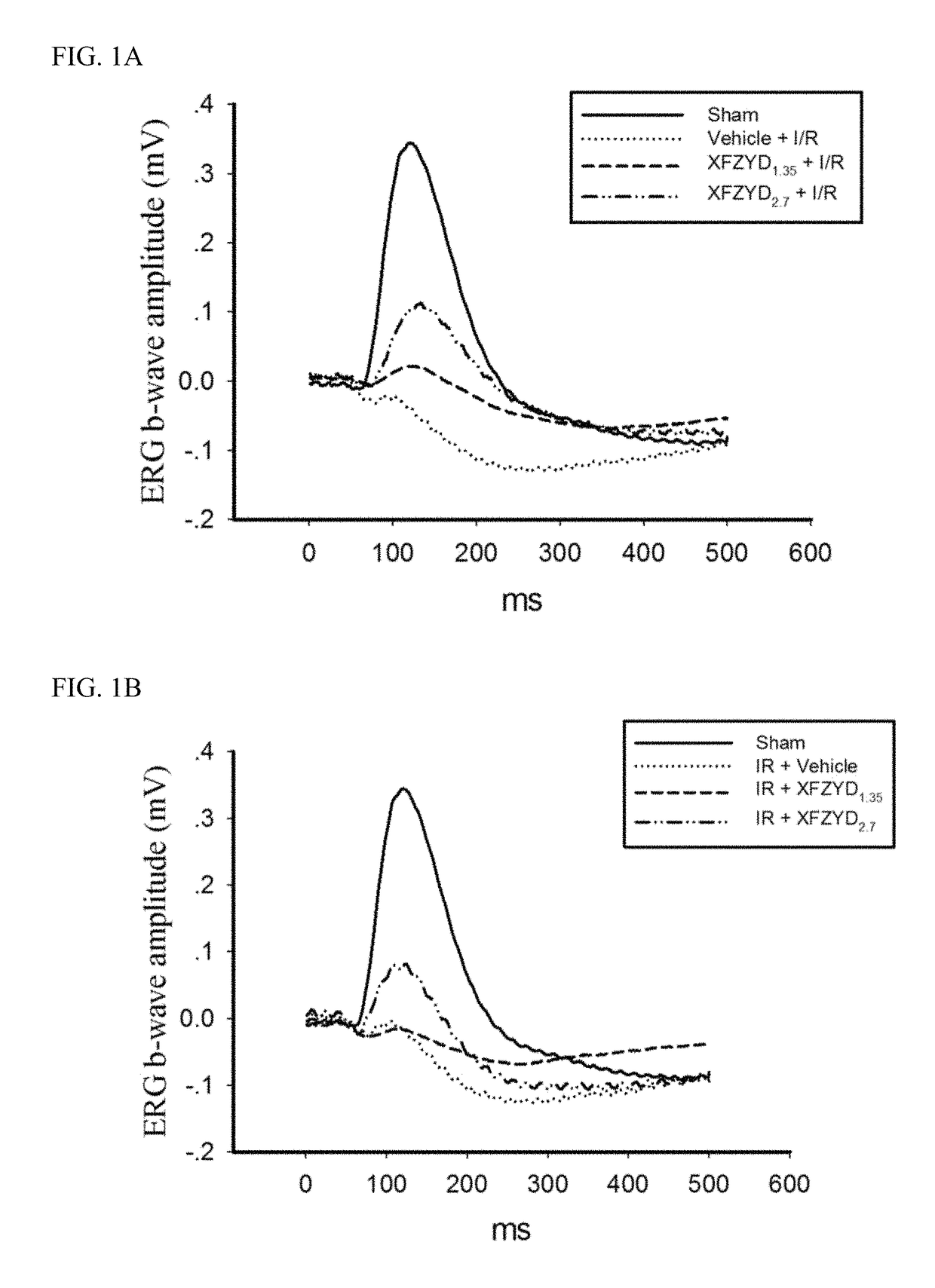
Inhibitors of PARP Activity and Uses Thereof
The present invention relates to new inhibitors of the nucleic enzyme poly(adenosine 5′-diphospho-ribose) polymerase [“poly(ADP-ribose) polymerase” or “PARP”, which is also sometimes called “PARS” for poly(ADP-ribose) synthetase]. More particularly, the invention relates to the use of PARP inhibitors to prevent and / or treat tissue damage resulting from cell damage or death due to necrosis or apoptosis; neural tissue damage resulting from ischemia and reperfusion injury; neurological disorders and neurodegenerative diseases; to prevent or treat vascular stroke; to treat or prevent cardiovascular disorders; to treat other conditions and / or disorders such as age-related macular degeneration, AIDS and other immune senescence diseases, arthritis, atherosclerosis, cachexia, cancer, degenerative diseases of skeletal muscle involving replicative senescence, diabetes, head trauma, immune senescence, inflammatory bowel disorders (such as colitis and Crohn's disease), muscular dystrophy, osteoarthritis, osteoporosis, chronic and acute pain (such as neuropathic pain), renal failure, retinal ischemia, septic shock (such as endotoxic shock), and skin aging; to extend the lifespan and proliferative capacity of cells; to alter gene expression of senescent cells; or to radiosensitize hypoxic tumor cells.
Owner:CENT NAT DE LA RECHERCHE SCI
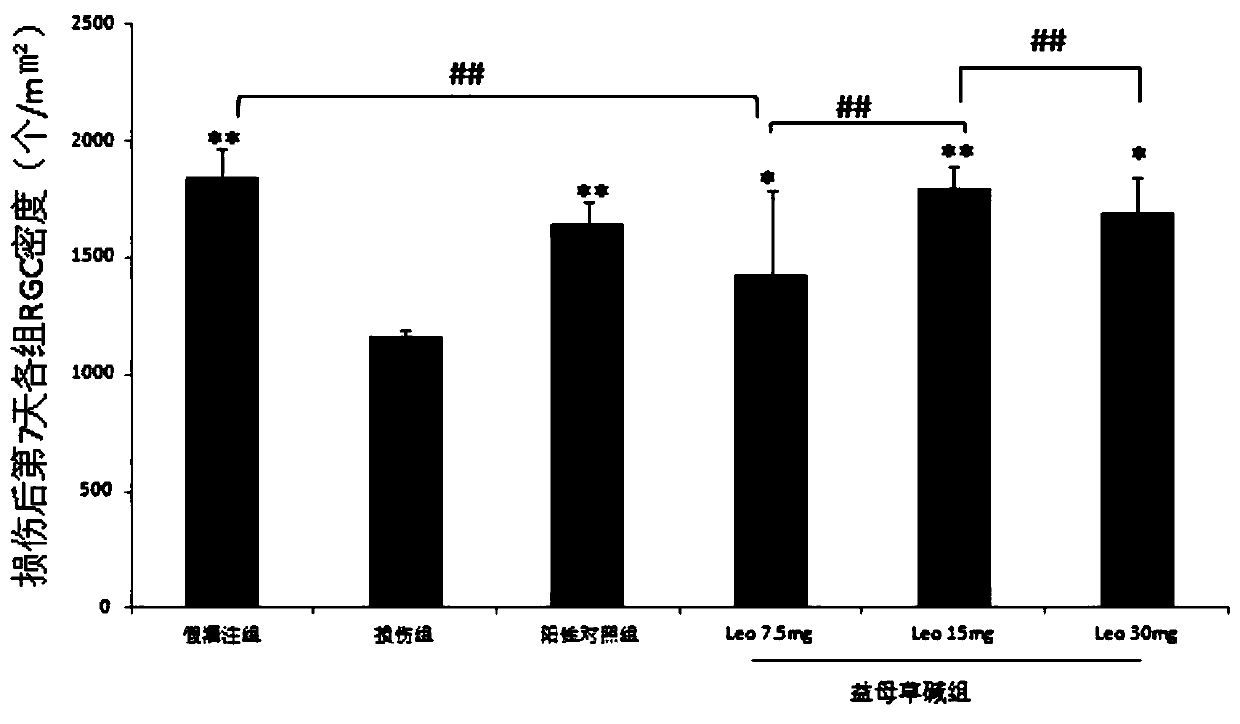
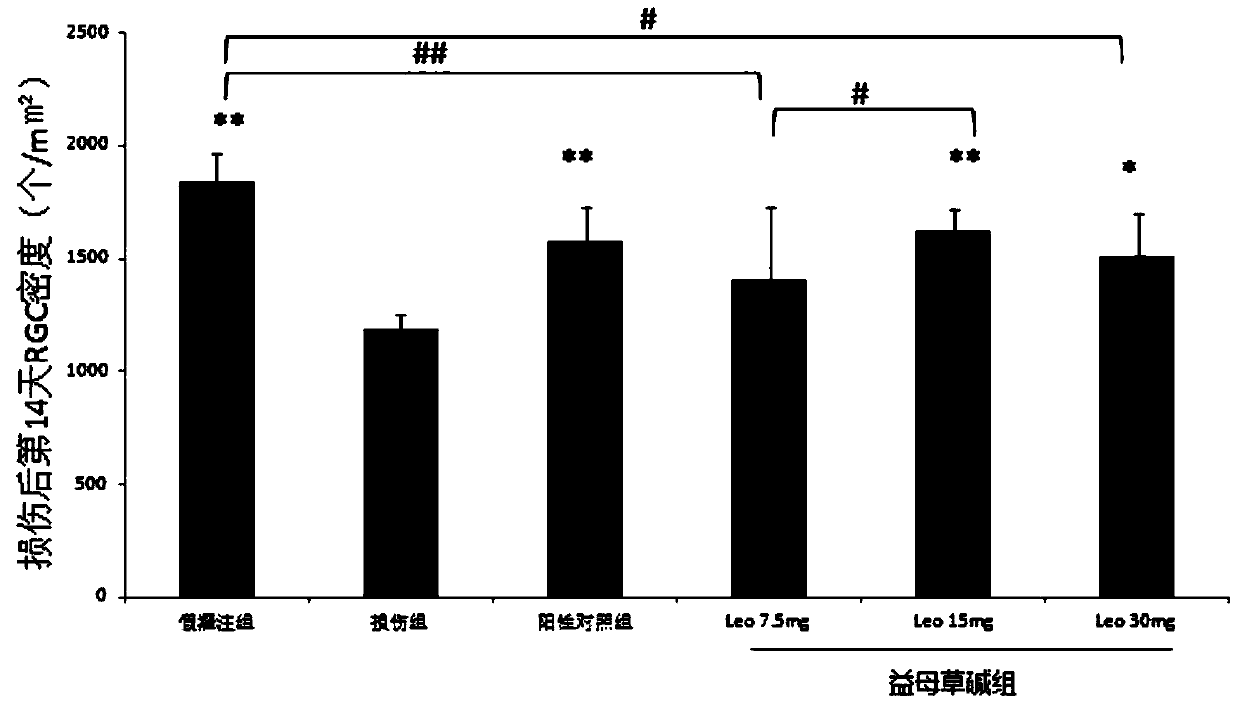
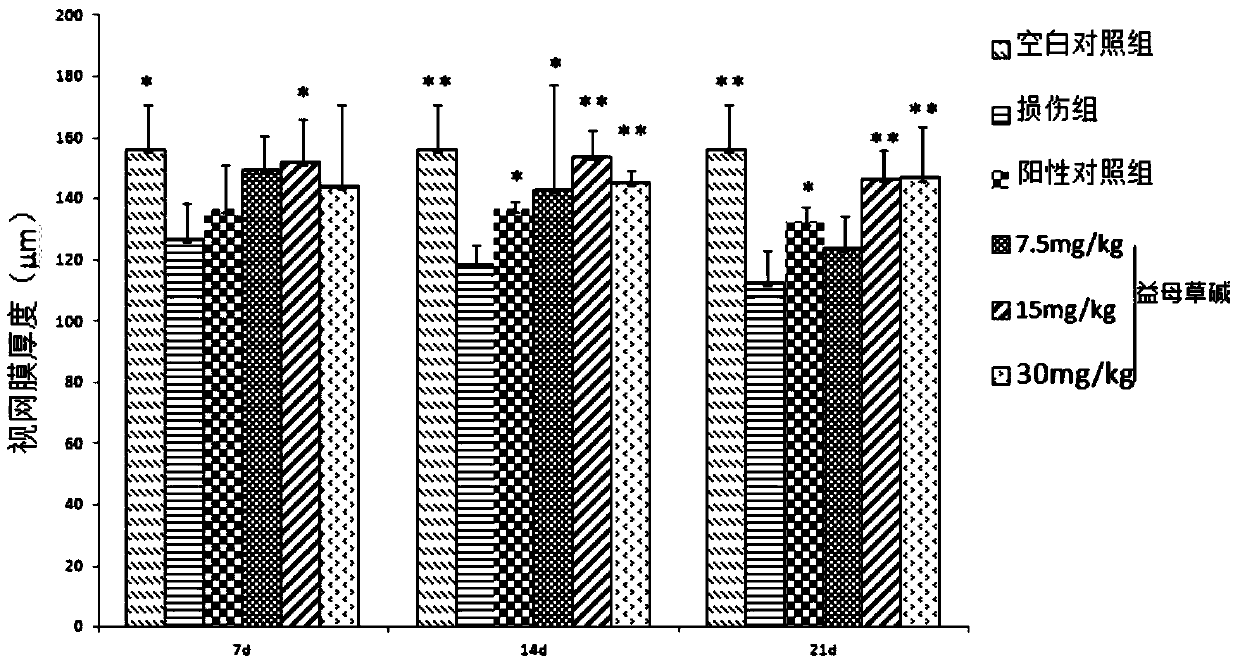
Use of Leonurine in the Preparation of Retinal Optic Nerve Protective Drugs
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
PARP inhibitors
The present invention relates to new inhibitors of the nucleic enzyme poly(adenosine 5′-diphospho-ribose) polymerase [“poly(ADP-ribose) polymerase” or “PARP”, which is also sometimes called “PARS” for poly(ADP-ribose) synthetase]. More particularly, the invention relates to the use of PARP inhibitors to prevent and / or treat tissue damage resulting from cell damage or death due to necrosis or apoptosis; neural tissue damage resulting from ischemia and reperfusion injury; neurological disorders and neurodegenerative diseases; to prevent or treat vascular stroke; to treat or prevent cardiovascular disorders; to treat other conditions and / or disorders such as age-related macular degeneration, AIDS and other immune senescence diseases, arthritis, atherosclerosis, cachexia, cancer, degenerative diseases of skeletal muscle involving replicative senescence, diabetes, head trauma, immune senescence, inflammatory bowel disorders (such as colitis and Crohn's disease), muscular dystrophy, osteoarthritis, osteoporosis, chronic and acute pain (such as neuropathic pain), renal failure, retinal ischemia, septic shock (such as endotoxic shock), and skin aging; to extend the lifespan and proliferative capacity of cells; to alter gene expression of senescent cells; or to radiosensitize hypoxic tumor cells.
Owner:CENT NAT DE LA RECHERCHE SCI
RNAi-MEDIATED INHIBITION OF IGF1R FOR TREATMENT OF OCULAR ANGIOGENESIS
RNA interference is provided for inhibition of IGF1R mRNA expression for treating patients with ocular angiogenesis, particularly for treating retinal edema, diabetic retinopathy, sequela associated with retinal ischemia, posterior segment neovascularization (PSNV), and neovascular glaucoma, and for treating patients at risk of developing such conditions.
Owner:ARROWHEAD RES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka.patsnap.com/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00001.png)
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka.patsnap.com/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00002.png)
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka.patsnap.com/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00003.png)