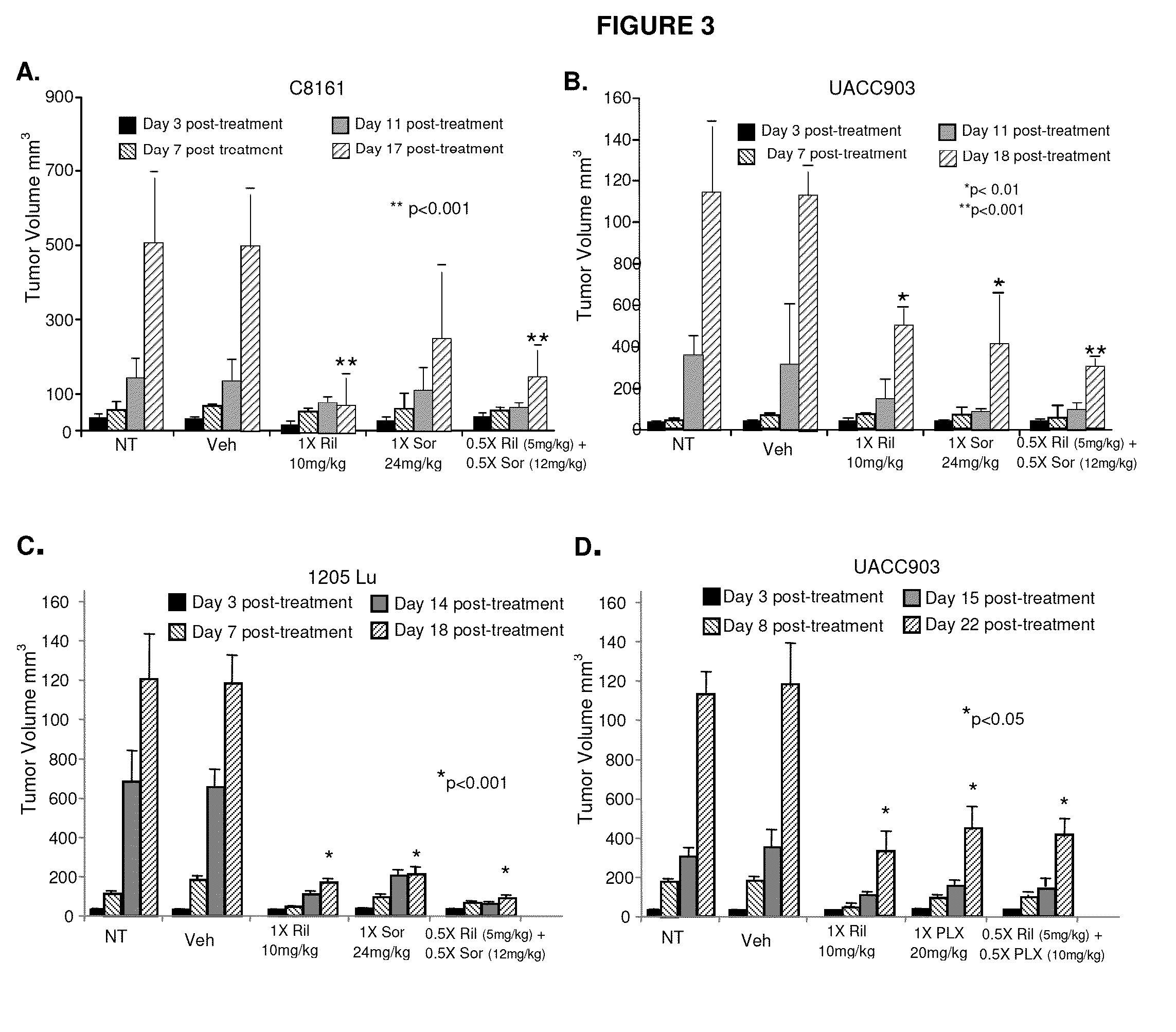
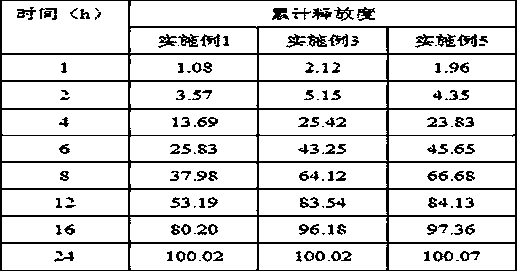
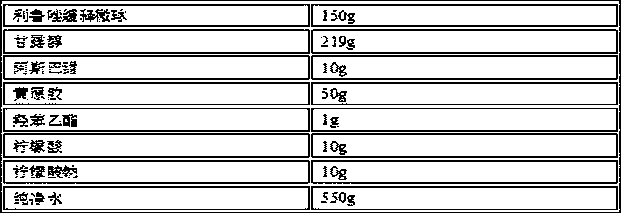
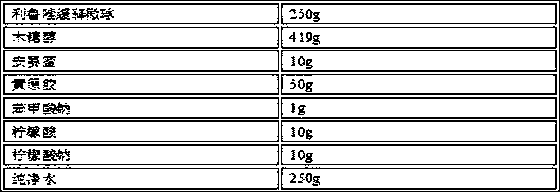
Patents
Literature
46 results about "Riluzole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
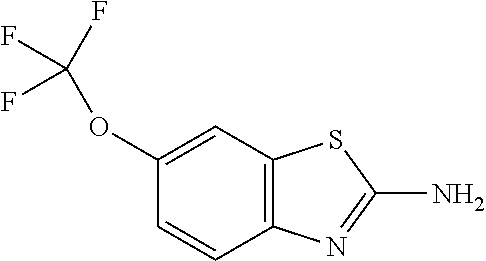
This medication is used to treat a certain type of nerve disease called amyotrophic lateral sclerosis (ALS, also commonly called Lou Gehrig's disease).
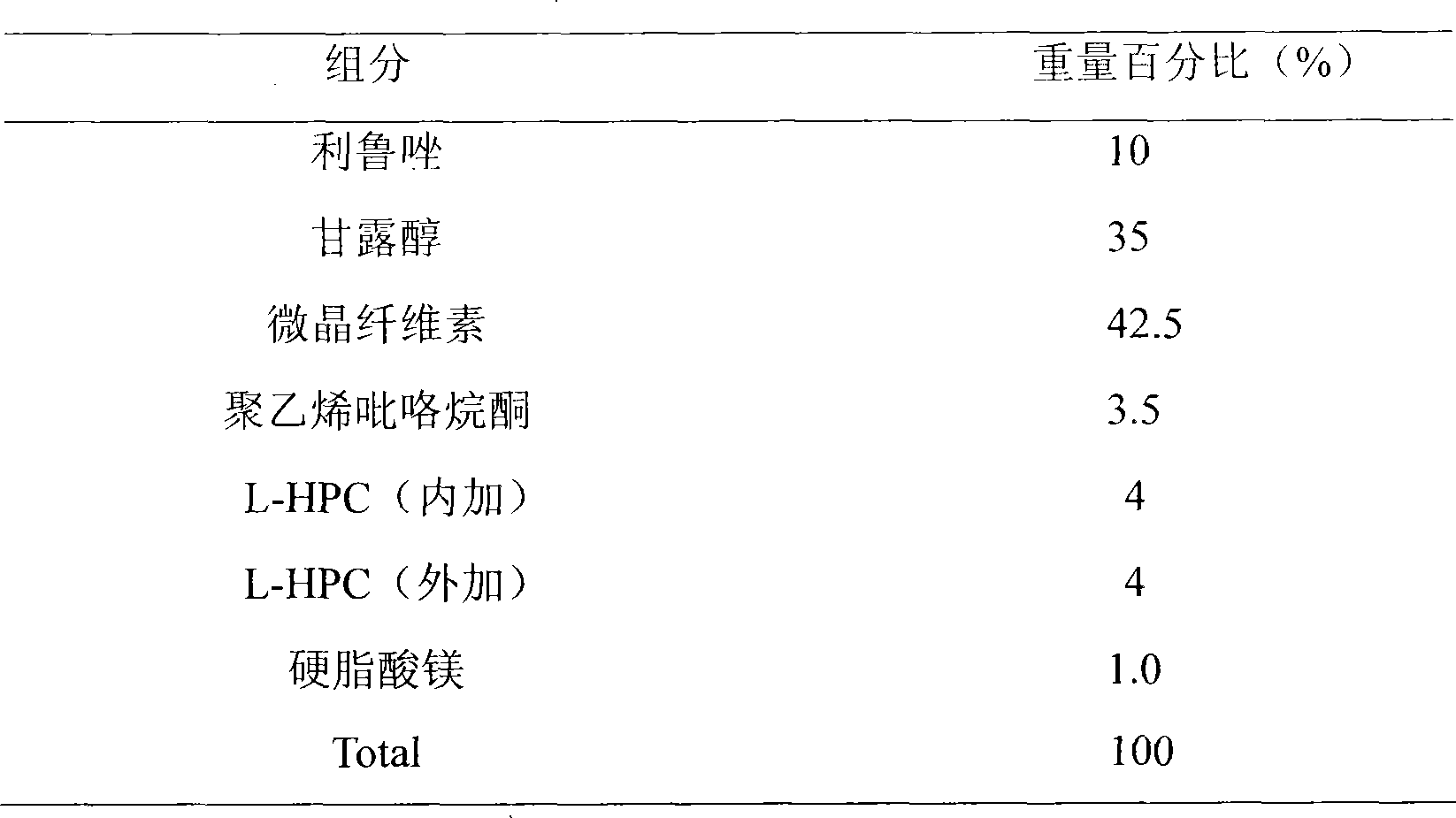
Medical composition containing riluzole
InactiveCN101390854AHigh dissolution rateEasy to takeOrganic active ingredientsNervous disorderChemical compositionBULK ACTIVE INGREDIENT
The invention discloses a stable medicinal combination which contains active ingredient riluzole and pharmaceutical auxiliary material. The medicine combination is mainly used for the clinical treatment of amyotrophic lateral sclerosis.
Owner:AVENTIS PHARMA HAINAN
Pharmaceutical for protection of motor nerve in patient with amyotrophic lateral sclerosis
InactiveUS20090149518A1Suppressing muscular weaknessSuppression of disabilityBiocideNervous disorderAnesthesiaObserved Survival
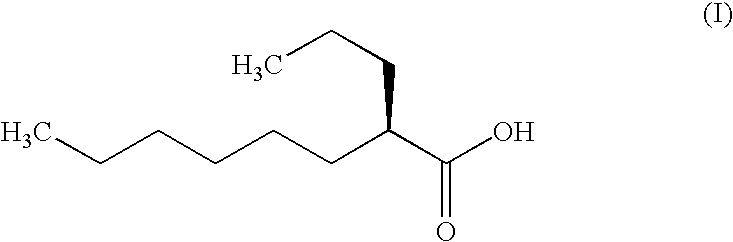
The present invention relates to an agent for protecting a motor nerve of a patient with amyotrophic lateral sclerosis, which comprises a combination of (a) (2R)-2-propyloctanoic acid or a salt thereof and (b) a therapeutic agent for amyotrophic lateral sclerosis such as riluzole. An agent comprising a combination of (a) (2R)-2-propyloctanoic acid which is orally administered once a day in an amount per dose of about 1200 mg and (b) riluzole which is orally administered twice a day in an amount per dose of about 50 mg is useful for protecting a motor nerve in a patient(s) with amyotrophic lateral sclerosis. The agent can also suppress the respiratory disability associated with the progression of the disease condition and improve the survival rate of the patient(s).
Owner:ONO PHARMA CO LTD
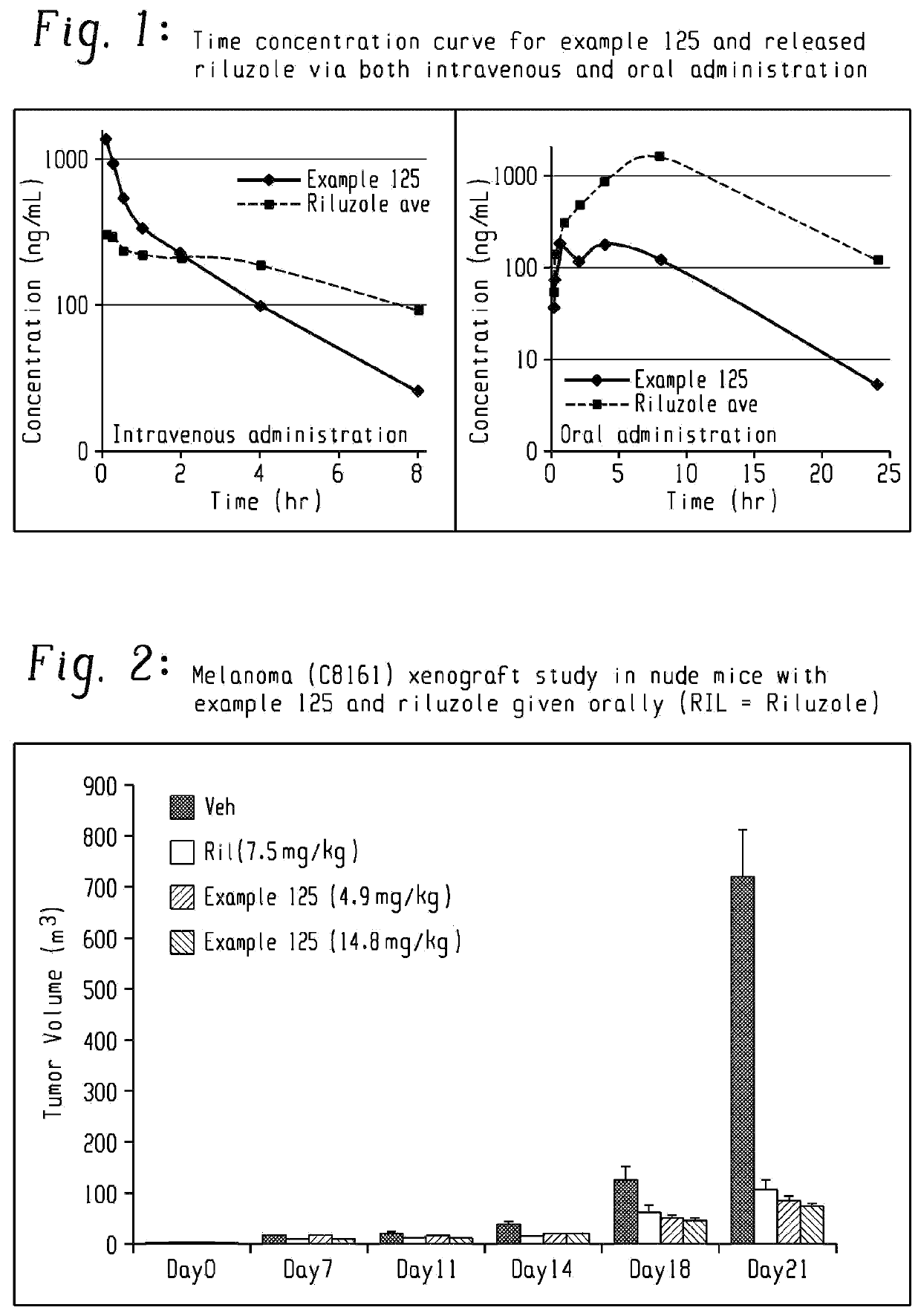
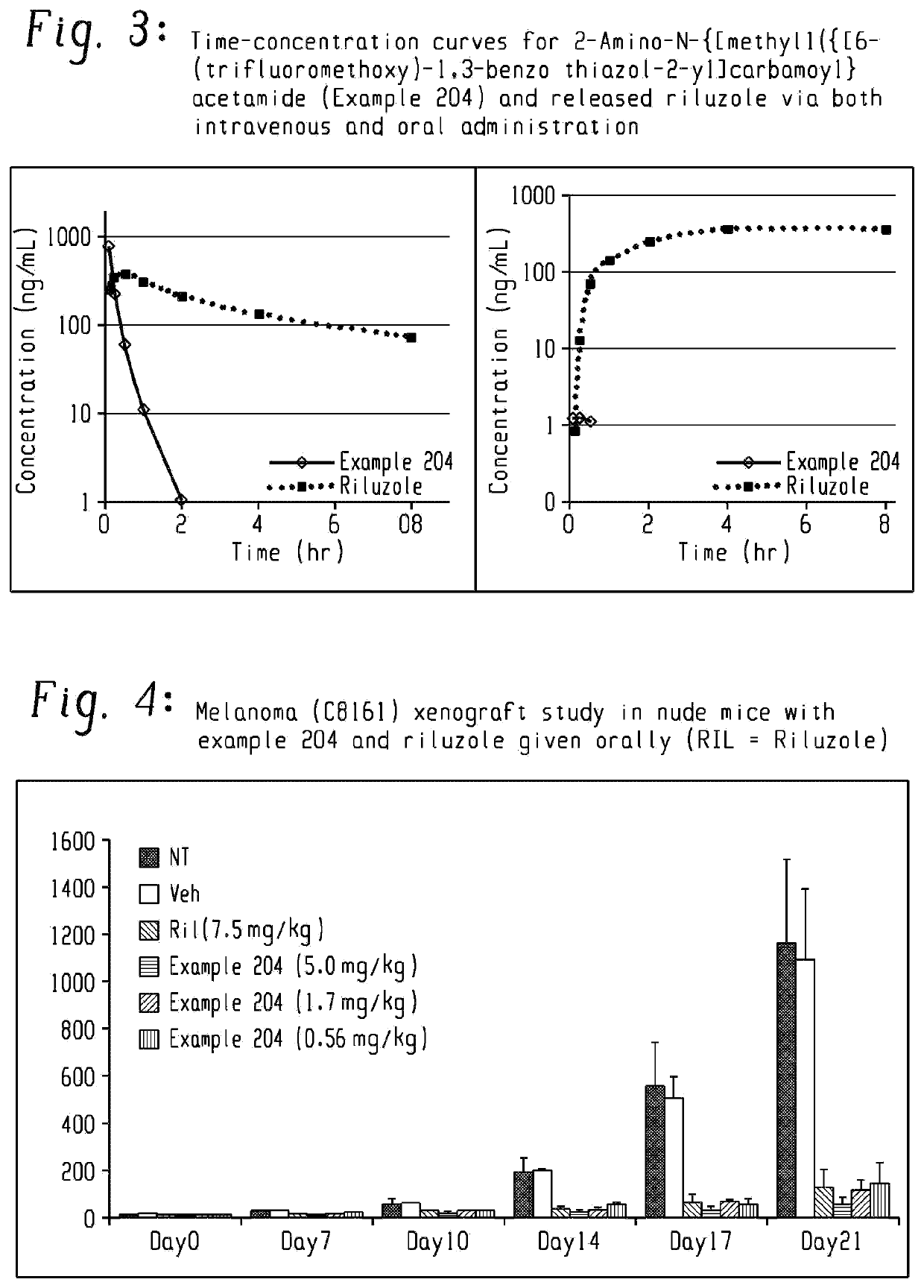
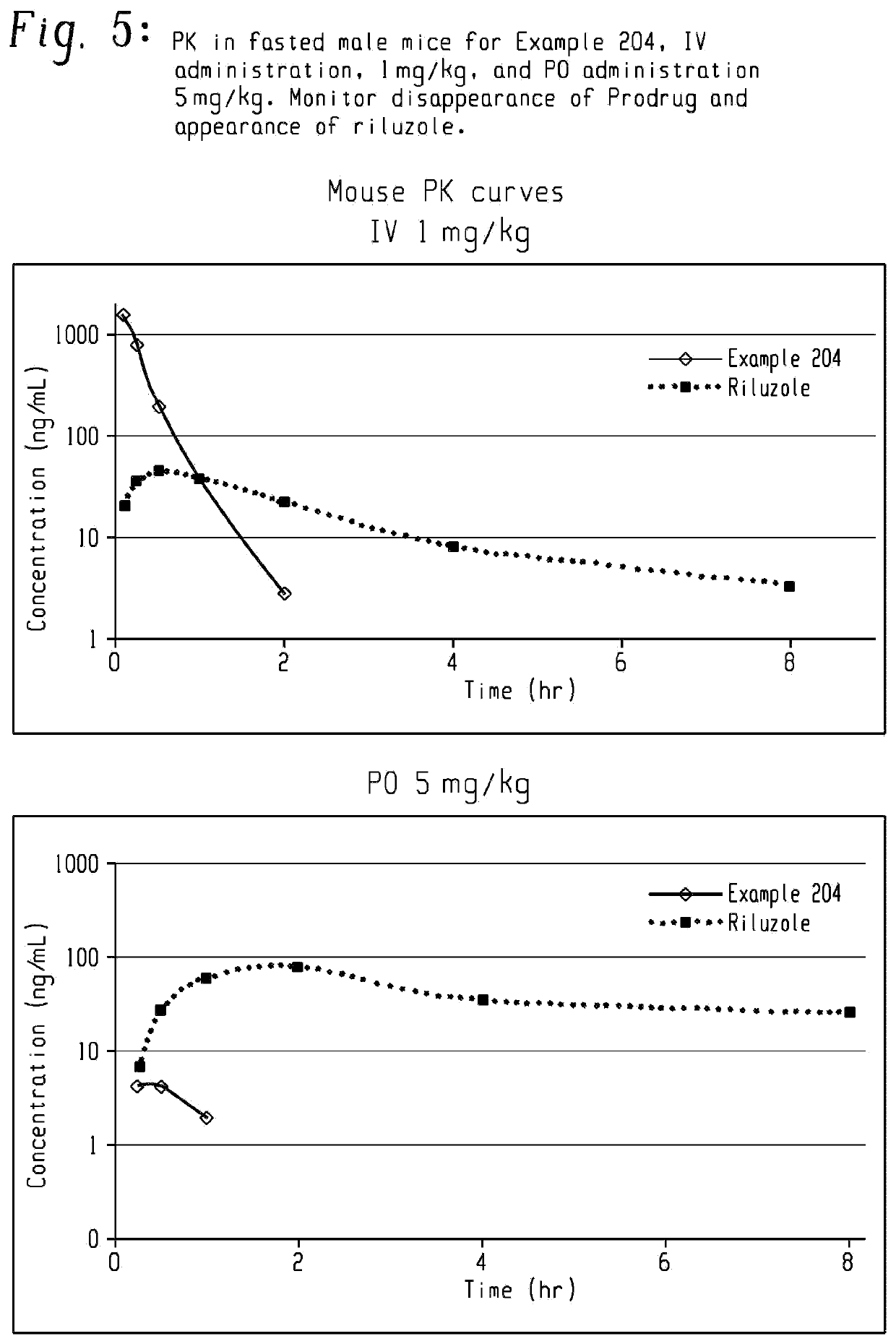
Pro-drugs of riluzole and their method of use for the treatment of amyotrophic lateral sclerosis
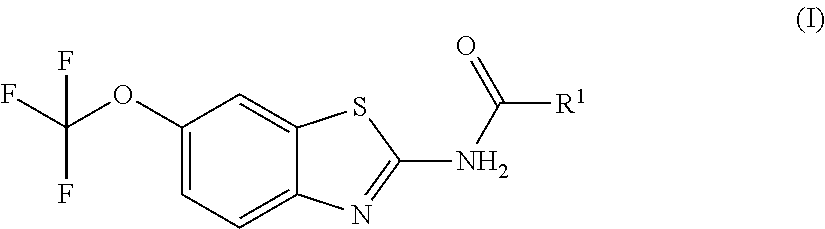
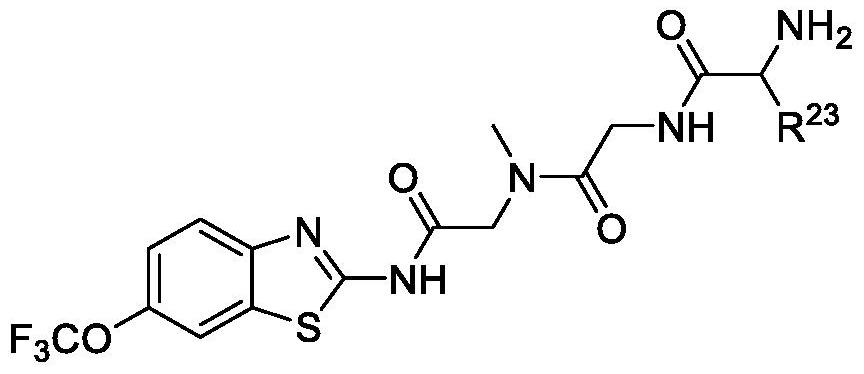
Pharmaceutical compositions of the invention include substituted riluzole pro drugs useful for the treatment of amyotrophic lateral sclerosis (ALS) and related disorders through the release of riluzole, especially to avoid patient to patient variability in first pass, hepatic metabolism promoted by Cyp 1A2. Pro-drugs of riluzole have enhanced stability to hepatic metabolism and are delivered into systemic circulation by oral administration, and then cleaved to release riluzole in the plasma via either an enzymatic or general biophysical release process. The invention further includes pro-drugs of riluzole useful for the treatment of disease states that can be treated with riluzole through the release of riluzole from a pro-drug agent.
Owner:BIOHAVEN PHARMA HLDG CO LTD
Combination Therapy With Glatiramer Acetate and Riluzole
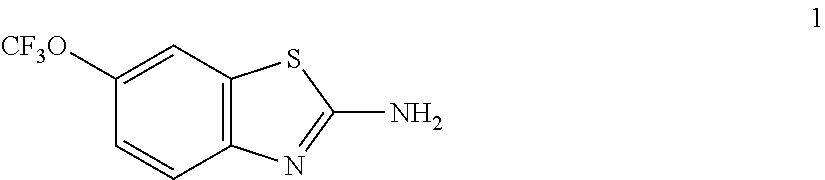
The subject invention provides a method of providing neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection comprising periodically administering to the subject an amount of glatiramer acetate and an amount of 2-amino-6-trifluoromethoxybenzathiazole, wherein the amounts when taken together are effective to provide neuroprotection to the central or peripheral nervous system of the subject. The subject invention also provides a package comprising glatiramer acetate, 2-amino-6-trifluorormethoxybenzothiazole and instructions for use together to provide neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection. Additionally, the subject invention provides a pharmaceutical composition comprising an amount of glatiramer acetate and an amount of 2-amino-6-trifluorormethoxybenzothiazole, wherein the amounts when taken together are effective to provide neuroprotection to the central or peripheral nervous system of the subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of 2-amino-6-trifluorormethoxybenzothiazole, which combination is useful to provide neuroprotection to the central or peripheral nervous system of the subject. In addition, the combination therapy may be used to treat a subject afflicted with multiple sclerosis or one afflicted with amyotrophic lateral sclerosis.
Owner:TEVA PHARM USA INC
Pharmaceutical formulation for treating muscular atrophy lateral sclerosis and preparing method
InactiveCN101293043AStrong targetingGood curative effectMuscular disorderNeuromuscular disorderSide effectTreatment effect
The invention pertains to the field of traditional Chinese medicine, in particular to a medicine preparation for treating amyotrophic lateral sclerosis and a preparation method thereof. The medicine preparation adopts the treatment principles of benefiting qi, nourishing yin and reinforcing yang, the traditional Chinese medicine compound preparation is prepared by taking pharmaceutical raw materials of membranous milkvetch root, dried rehmannia root, epimedium herb, medicinal Indianmulberry root and common macrocarpium fruit; the clinical studies show that the medicine preparation can significantly reduce the clinical integral of the traditional Chinese medicine symptoms of flaccidity diseases and improve the clinical symptoms of the patients with the flaccidity diseases / ALS; compared with the prior art, the treatment effects are significantly improved, furthermore, the medicine preparation is safe, reliable and has no nausea, vomit, decreased appetite, abdominal distension or other evident common side effects of riluzole.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
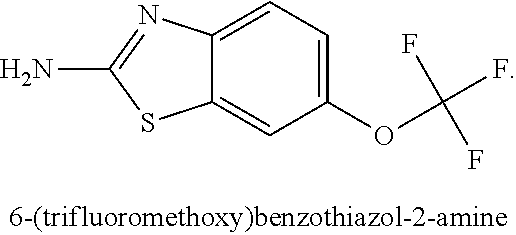
Use of riluzole for the treatment of multiple sclerosis
InactiveUS6872739B1Useful in preparationOrganic active ingredientsBiocidePharmacologyMultiple sclerosis
Methods for the treatment of multiple sclerosis are disclosed comprising administering 6-(triflouromethoxy)-2-benzothiazolamine or a salt thereof.
Owner:VER VOOR CHRISTELIJK WETENSHAPPELIKJK ONDERWIJS
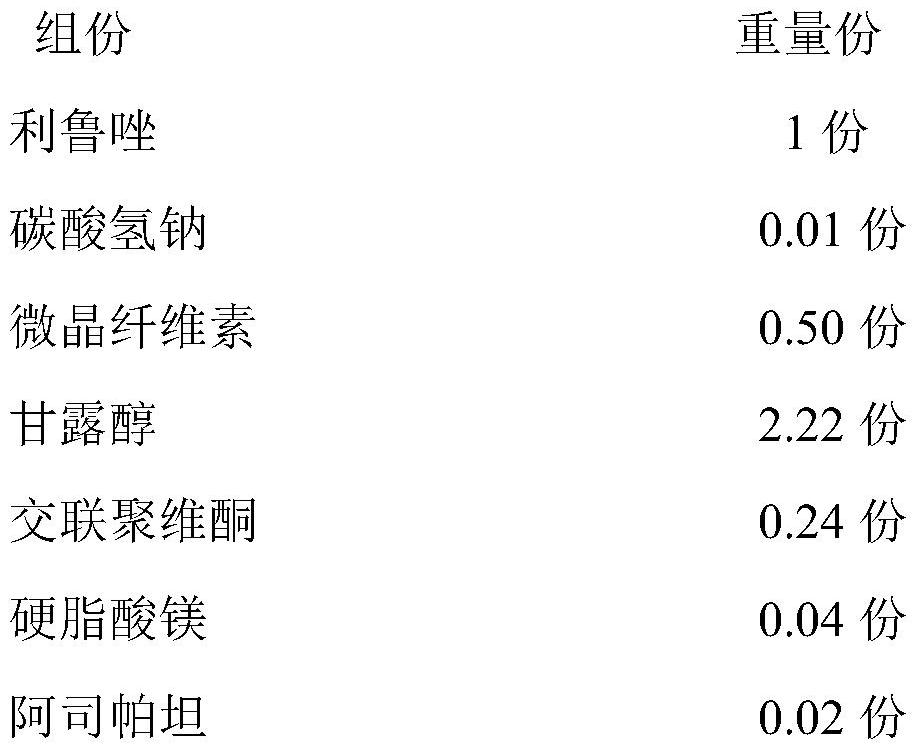
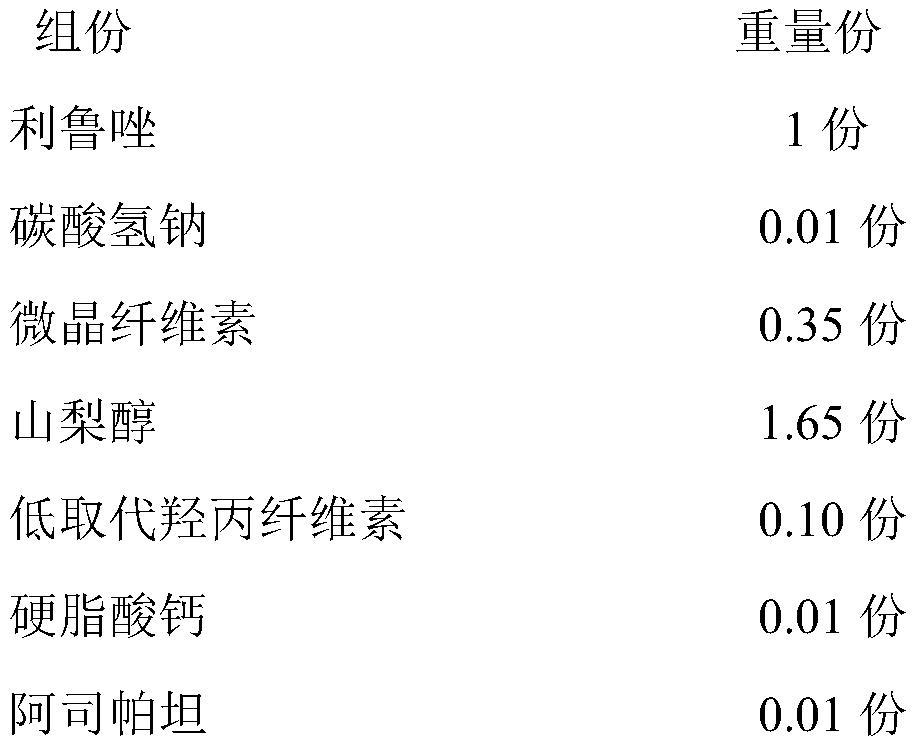
Riluzole orally disintegrating tablet and preparation method thereof
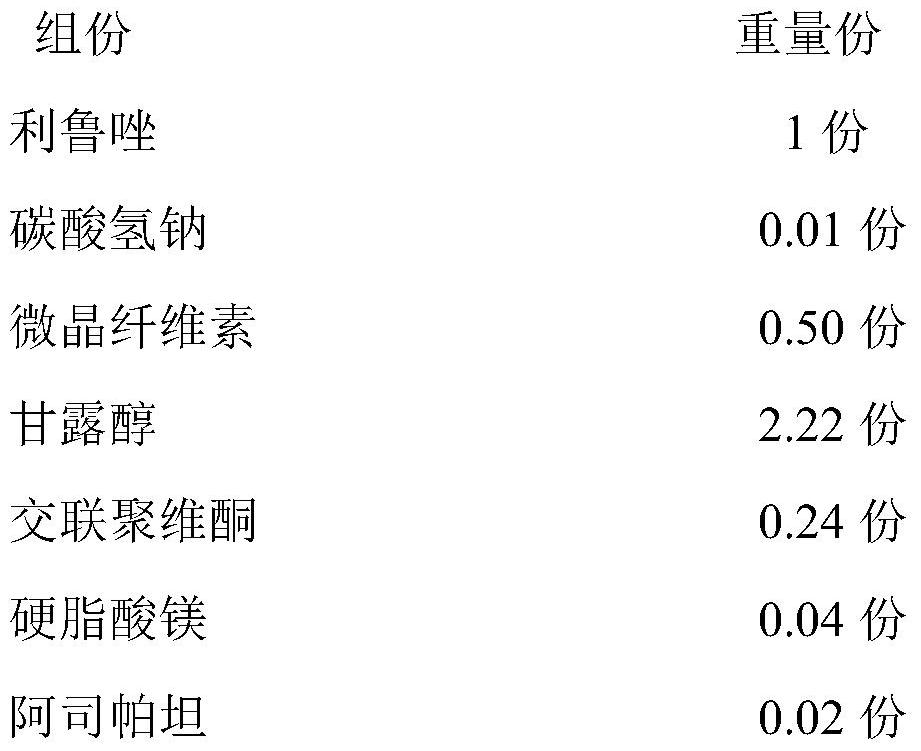
ActiveCN111821289AMeet the quality standard requirementsImprove quality stabilityOrganic active ingredientsAntineoplastic agentsSodium bicarbonateOrally disintegrating tablet
The invention relates to a riluzole orally disintegrating tablet and a preparation method thereof, and belongs to the field of preparations. The tablet is prepared from riluzole, sodium bicarbonate, afilling agent, a disintegrating agent, a lubricating agent and a flavoring agent. The preparation method comprises the following steps of (1) weighing riluzole, sodium bicarbonate and filler according to prescription amounts, and uniformly mixing the riluzole, the sodium bicarbonate and the filler with 50% of the disintegrating agent according to prescription amounts for later use; 2) adding themixture obtained in the step 1) into an ethanol water solution, granulating, drying, and sieving to obtain dry granules for later use; and 3) adding 50% of a prescription amount of the disintegratingagent and a prescription amount of the flavoring agent into the dry particles obtained in the step 2, uniformly mixing, adding a prescription amount of the lubricant, uniformly mixing, and pressing toobtain the riluzole orally disintegrating tablet. The orally disintegrating tablet prepared by the invention has the advantages of short disintegration time limit, high dissolution rate and no gravelfeeling, meets the quality standard requirement of the orally disintegrating tablet, and is suitable for industrial scale-up production.
Owner:LUNAN PHARMA GROUP CORPORATION
Treatment of dyskinesias and Parkinson's disease with riluzole and levodopa
This invention relates to pharmaceutical compositions of riluzole in combination with levodopa and methods of treating Parkinson's disease and alleviating levodopa-induced dyskinesia and tardive dyskinesia therewith. Pharmaceutical compositions of riluzole in combination with an antipsychotic drug are also provided for use in the treatment of behavioral and psychiatric disorders treatable with an antipsychotic drug.
Owner:NST NEUROSURVIVAL TECH +3
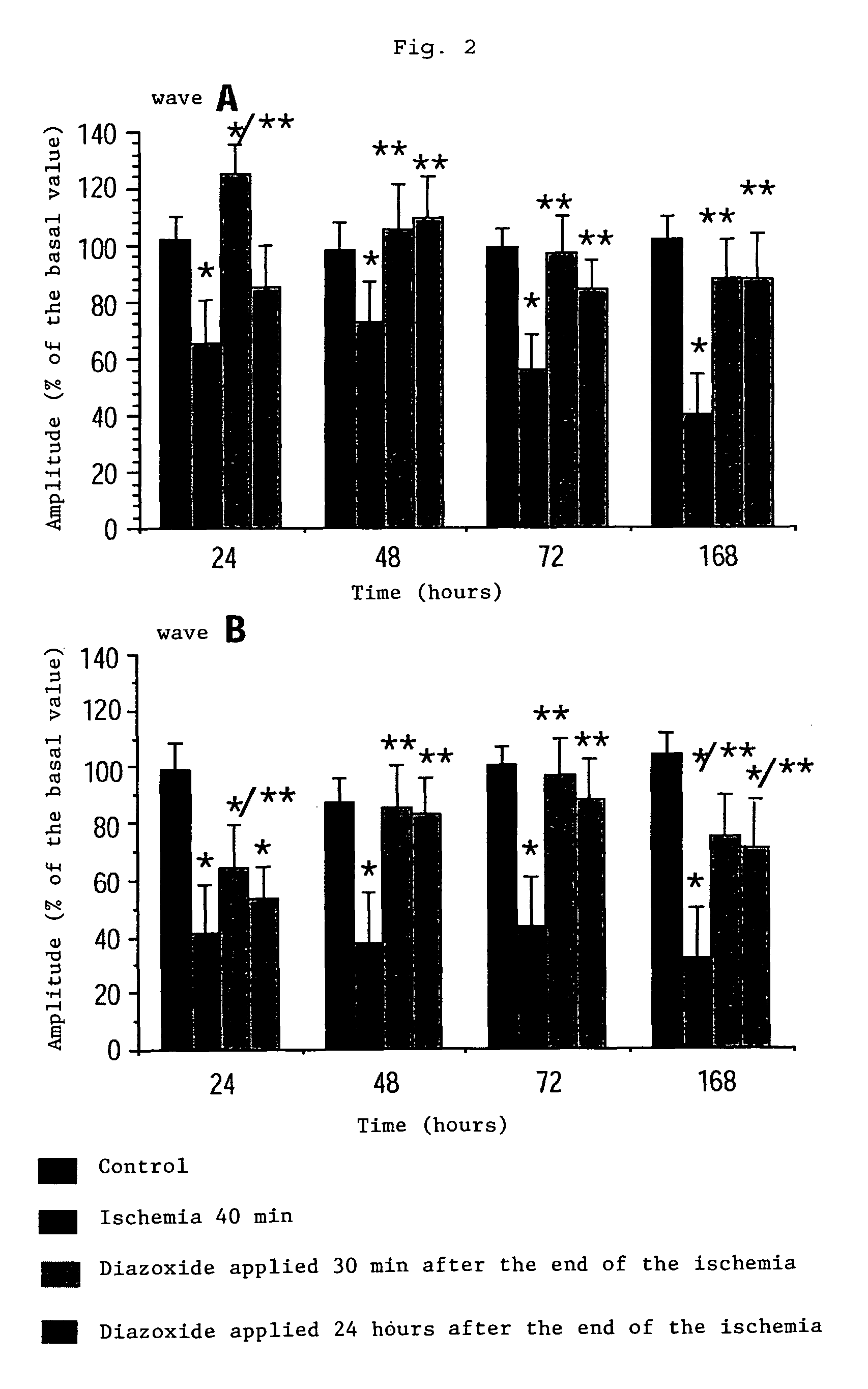
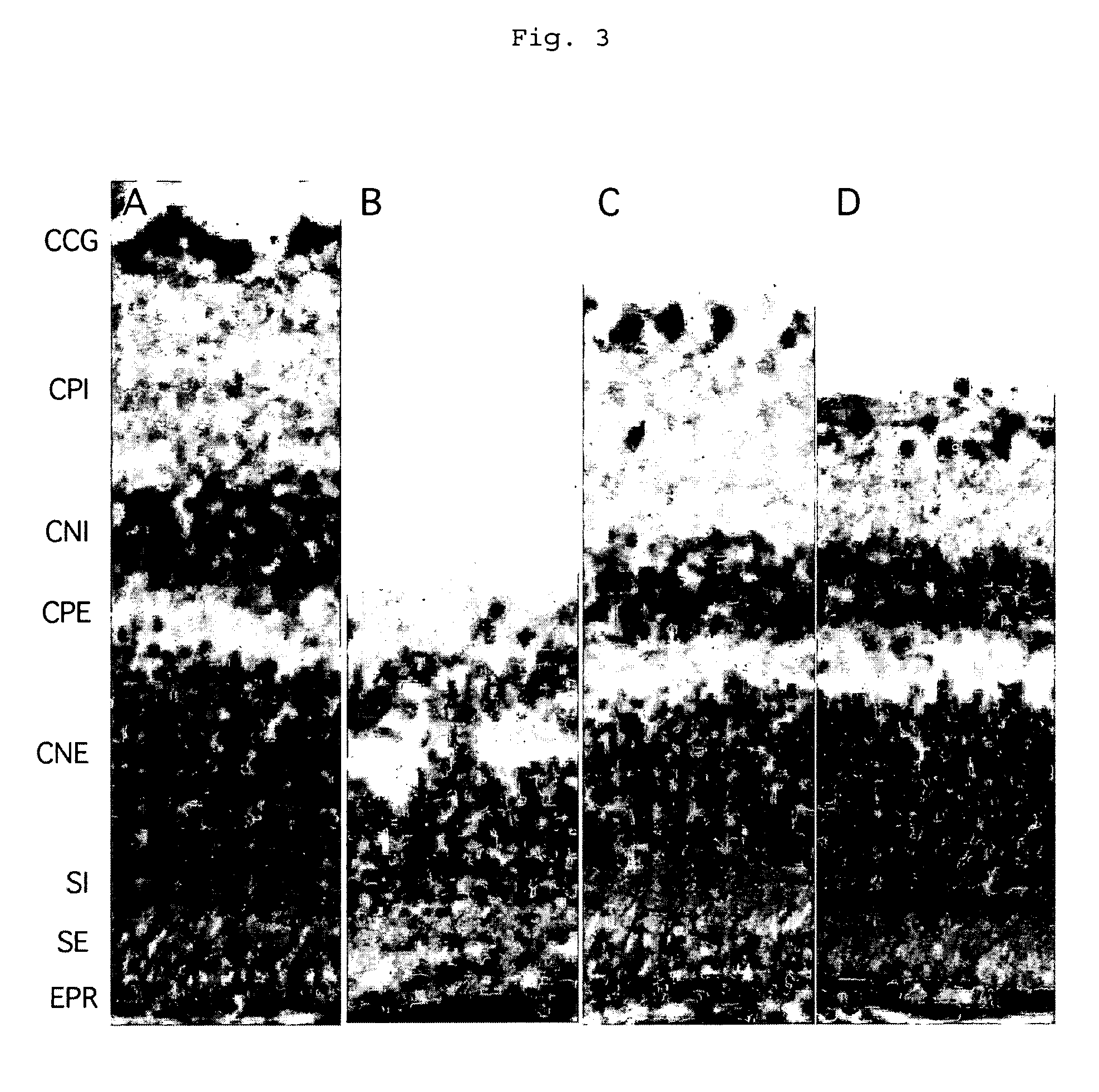
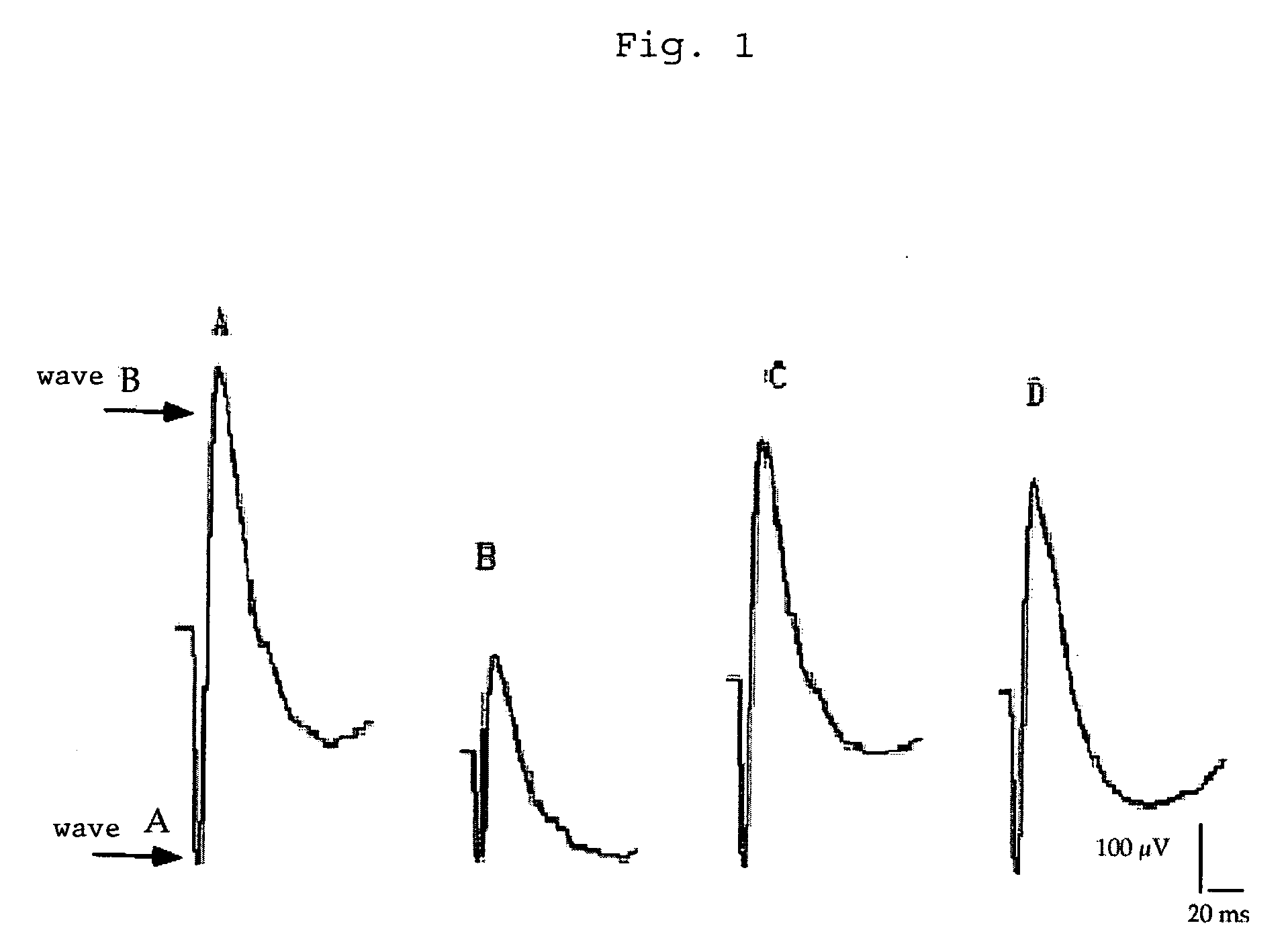
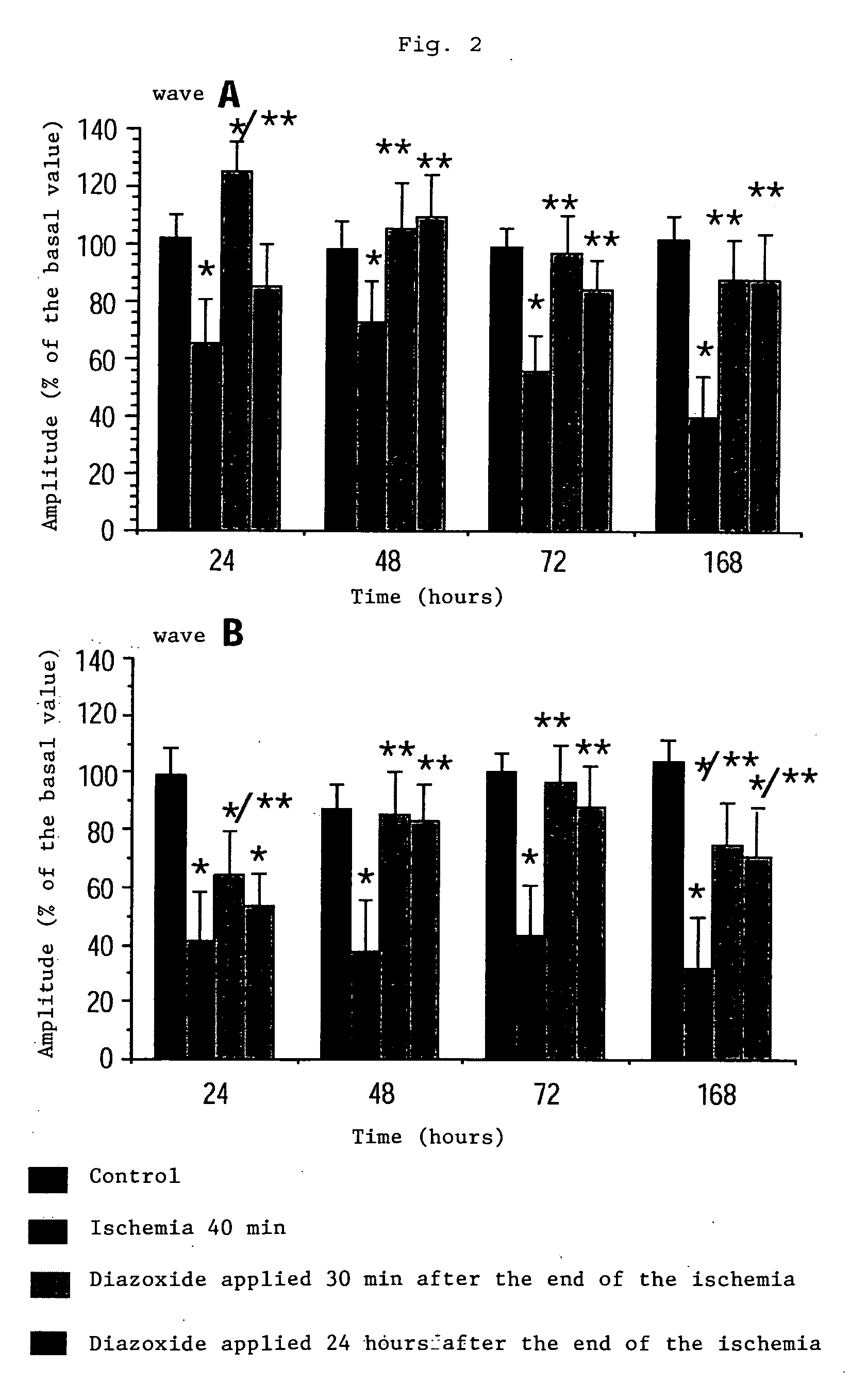
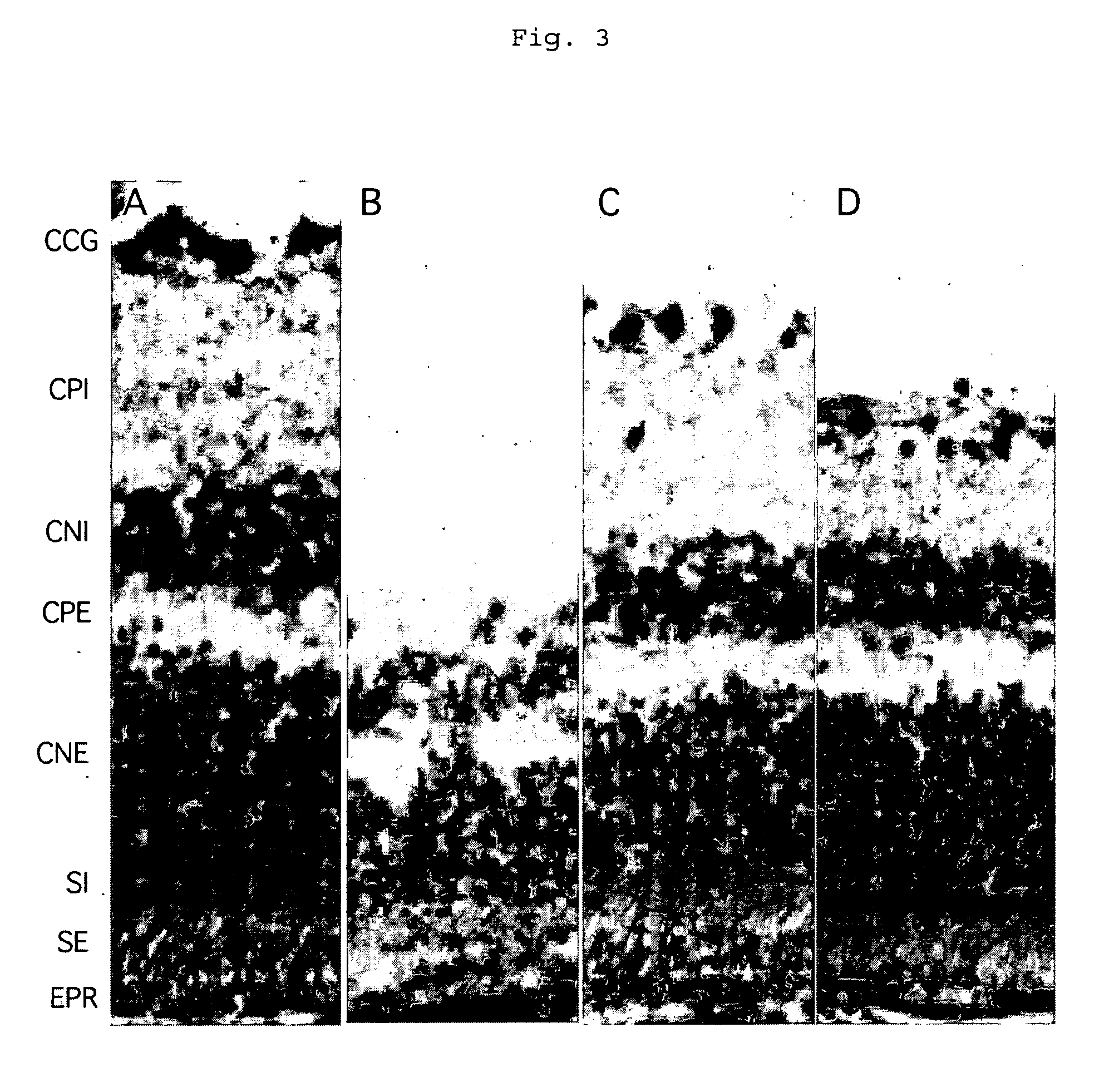
Method of treatment of retinal ischemia with diazoxide
A composition including diazoxide (7-chloro-3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxide) for the treatment and / or prevention of retinal ischemia and of diseases associated with retinal ischemia. The composition can also contain riluzole, a derivative active in neuroprotection of the latter, or a pharmaceutically acceptable salt of the latter.
Owner:CENT NAT DE LA RECHERCHE SCI +1
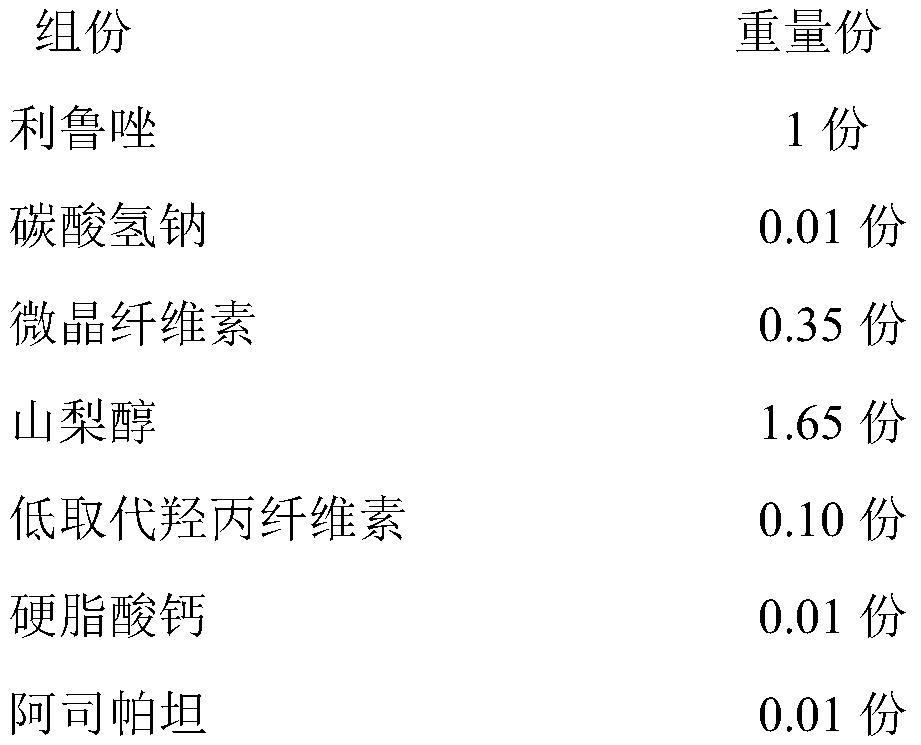
Riluzole tablets and preparation method thereof
ActiveCN105030708AAdvantages and Significant AdvancementsGood dissolution effectOrganic active ingredientsNervous disorderMedicinePolyethylene glycol
The invention provides riluzole tablets and a preparation method thereof. The riluzole tablets comprise riluzole, polyethylene glycol, an N-vinyl pyrrolidone / vinyl acetate linear copolymer, a filling agent, a disintegrating agent and a lubricating agent. According to the invention, riluzole and polyethylene glycol are melted firstly, then the melted riluzole and polyethylene glycol are added into an ethanol solution of the N-vinyl pyrrolidone / vinyl acetate linear copolymer, and finally the corresponding filling agent, the disintegrating agent and the lubricating agent are added for tabletting, so that the riluzole tablets are obtained. The dissolution effect of the prepared riluzole tablets is improved obviously and can reach 80% or above with 10 min, the long-term storage stability is good, the preparation process is simple, complex equipment is not required and industrial mass production is facilitated.
Owner:LUNAN BETTER PHARMA
Method of treatment of retinal ischemia with diazoxide
A composition including diazoxide (7-chloro-3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxide) for the treatment and / or prevention of retinal ischemia and of diseases associated with retinal ischemia. The composition can also contain riluzole, a derivative active in neuroprotection of the latter, or a pharmaceutically acceptable salt of the latter.
Owner:CENT NAT DE LA RECHERCHE SCI +1
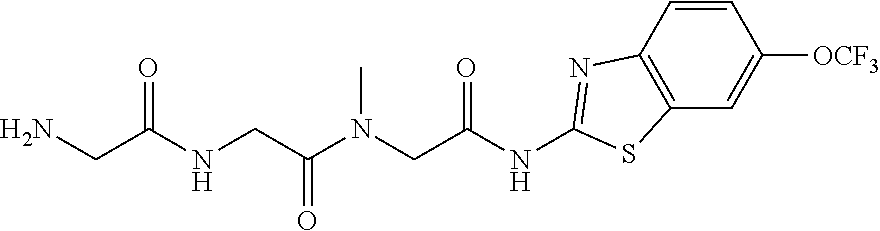
Riluzole prodrugs and their use
ActiveUS20180036290A1Increasing serotonin activityOrganic active ingredientsNervous disorderMelanomaOral medication
Pharmaceutical compositions of the invention include substituted riluzole prodrugs useful for the treatment of cancers including melanoma, breast cancer, brain cancer, and prostate cancer through the release of riluzole. Prodrugs of riluzole have enhanced stability to hepatic metabolism and are delivered into systemic circulation by oral administration, and then cleaved to release riluzole in the plasma via either an enzymatic or general biophysical release process.
Owner:BIOHAVEN THERAPEUTICS LTD
Use of glutamate modulating agents with immunotherapies to treat cancer
PendingUS20190175731A1Effective treatmentImprove responseOrganic active ingredientsTripeptide ingredientsImmunotherapeutic agentCTLA4 Protein
Disclosed are methods of treating cancer using a combination of an immunotherapeutic agent, such as, for example, a PD-1, PD-L1 or CTLA-4 checkpoint inhibitor, and a glutamate modulating agent such as riluzole or trigriluzole. Pharmaceutical compositions and kits including the immunotherapeutic agents and glutamate modulating agents are also disclosed.
Owner:BIOHAVEN THERAPEUTICS LTD
Therapeutic combinations containing riluzole
InactiveUS20130116274A1Convenient treatmentBiocideAnimal repellantsMelanomaMetabotropic glutamate receptor 1
Disclosed is a method of treating melanoma in a mammal comprising administering (a) a therapeutically effective amount of an inhibitor of metabotropic glutamate receptor 1 (GRM1); and (b) a therapeutically effective amount of an inhibitor of at least one downstream signaling target of GRM1. Also disclosed are compositions and kits for treating melanoma comprising (a) a therapeutically effective amount of an inhibitor of GRM1; and (b) a therapeutically effective amount of an inhibitor of at least one downstream signaling target of GRM1.
Owner:RUTGERS THE STATE UNIV
Riluzole sustained-release oral-administration suspension
InactiveCN111437256AStable blood concentrationOrganic active ingredientsNervous disorderSuspending AgentsDrug administration
The invention belongs to the technical field of pharmaceutical preparations and discloses a Riluzole sustained-release oral-administration suspension. The Riluzole sustained-release oral-administration suspension is used for simultaneously solving problems in smooth release of drugs and compliance of drug administration of people suffering from dysphagia such as the elderly and children. The disclosed sustained-release suspension is prepared through dispersing Riluzole sustained-release microspheres in a solution containing a diluent, a flavoring agent, a suspending agent, a preservative and apH buffering agent, wherein the Riluzole sustained-release microspheres are prepared through dissolving Riluzole in an aqueous dispersion of ethyl cellulose and carrying out spray drying. Compared with common tablets, capsules and oral-administration suspensions on the market at present, the Riluzole sustained-release oral-administration suspension disclosed by the invention has the advantages that drug release is smooth, the number of times of drug administration is reduced, the quality is stable, the mouth feel is good, the compliance of drug administration is high, and the Riluzole sustained-release oral-administration suspension is applicable to the people suffering from the dysphagia such as the elderly and the children.
Owner:BEIJING VENTUREPHARM BIOTECH
Riluzole aqueous suspensions
Physically and chemically stable aqueous oral suspensions of riluzole and manufacturing methods thereof. The suspensions contain riluzole in particle form and at least a wetting agent, preferably a surfactant. Riluzole is present in amounts from about 0.1% to about 20% w / v and has an average particle size lower than 200 μm. The suspensions are devoid of the known local (mouth) anaesthetic effects of riluzole.
Owner:ITALFARMACO SPA
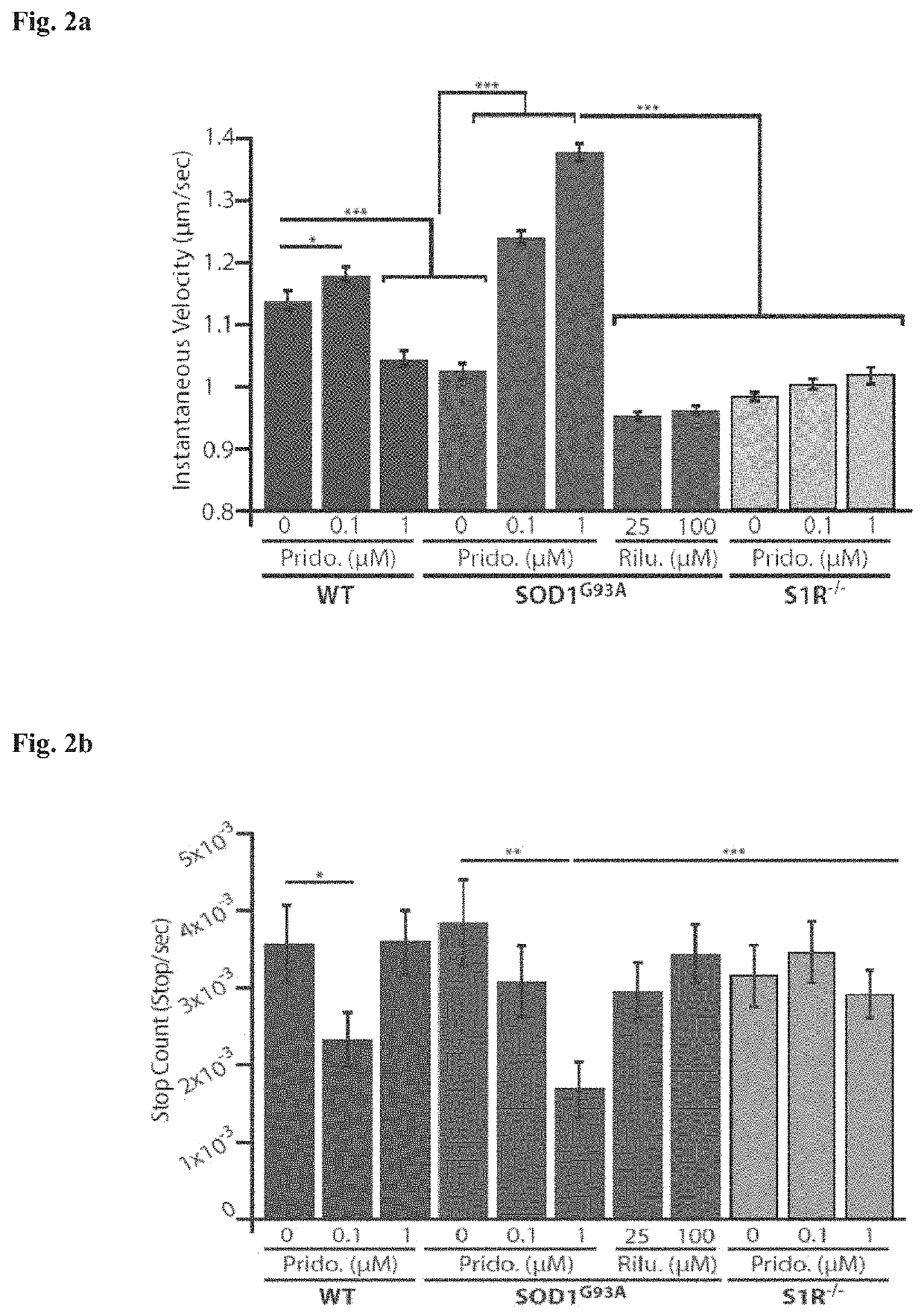
Method of treating amyotrophic lateral sclerosis with pridopidine
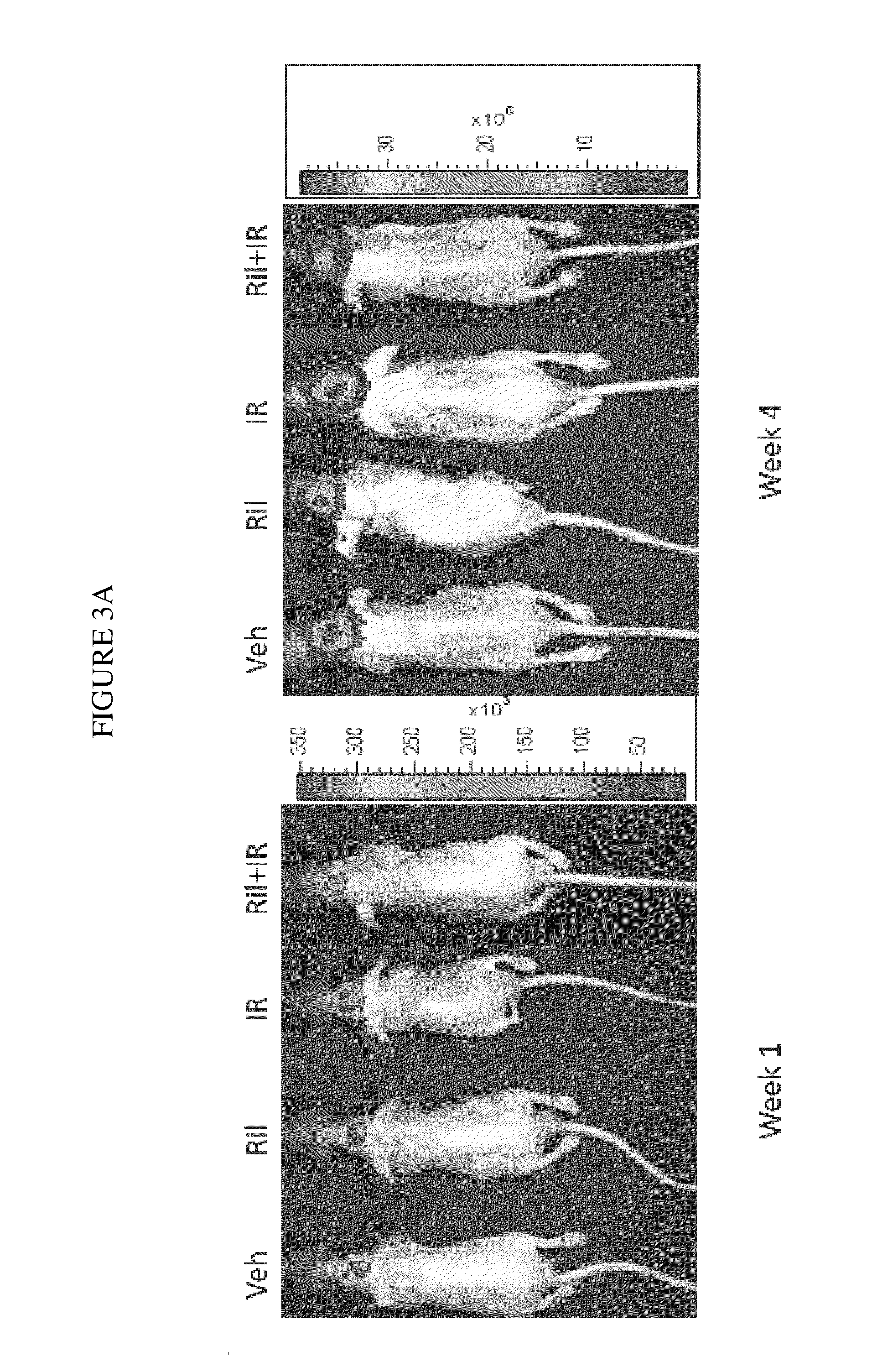
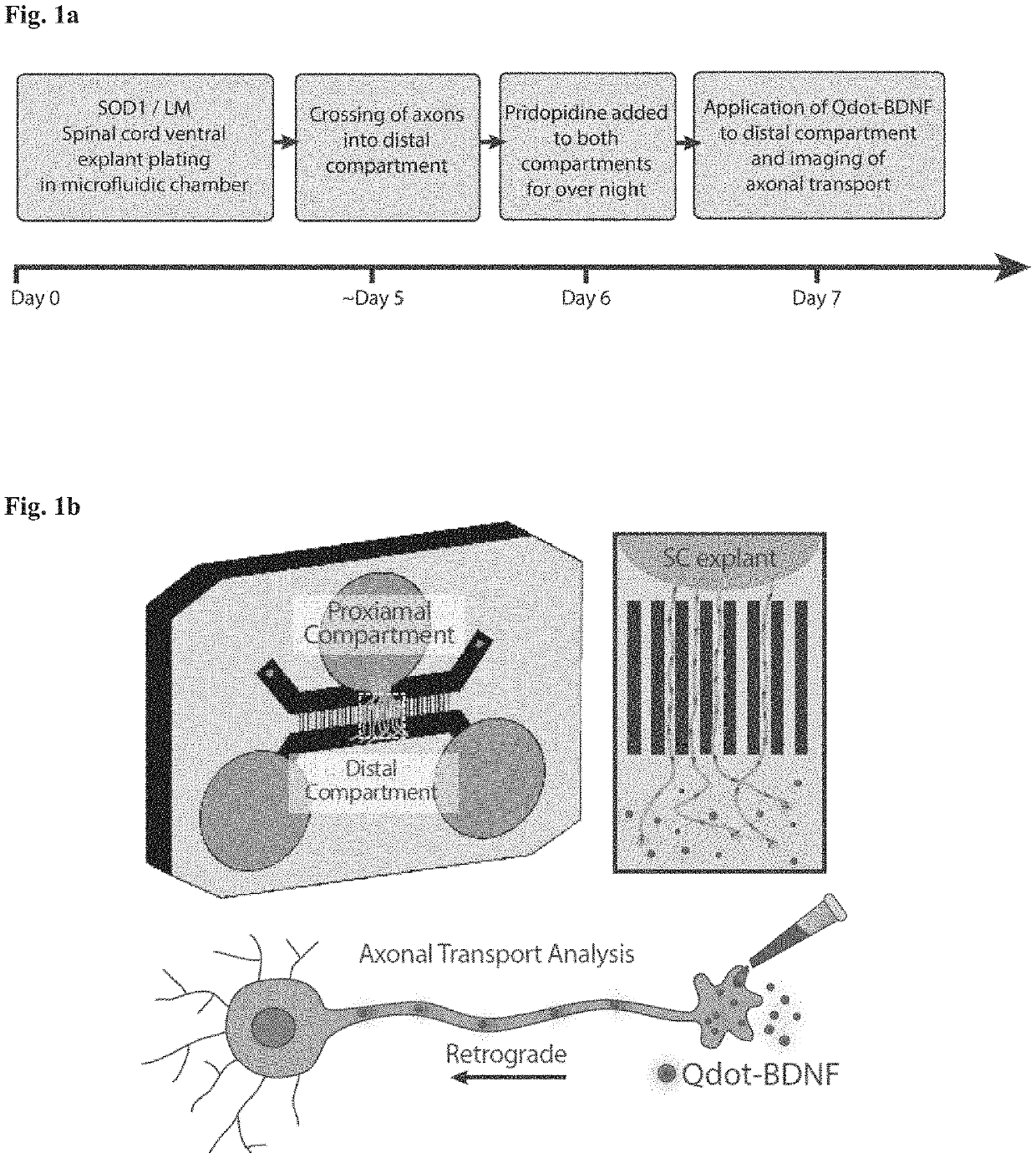
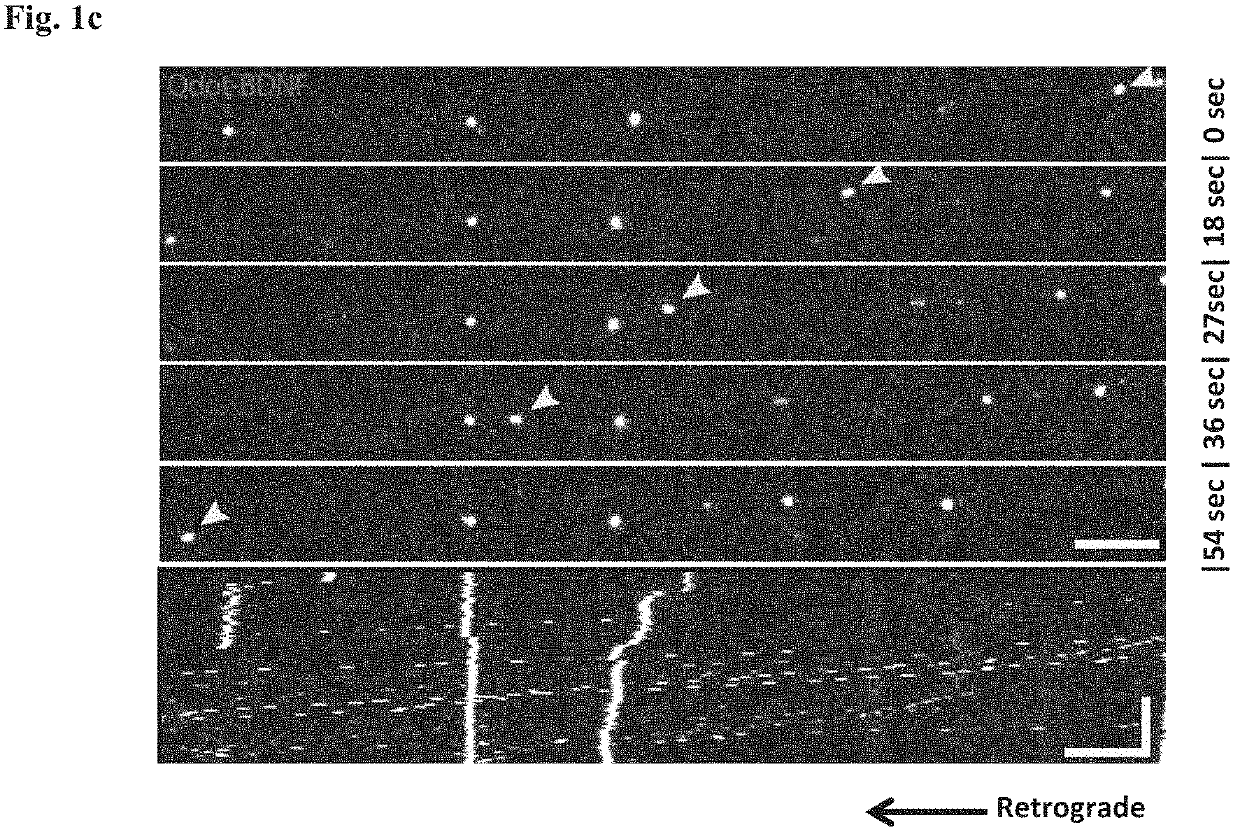
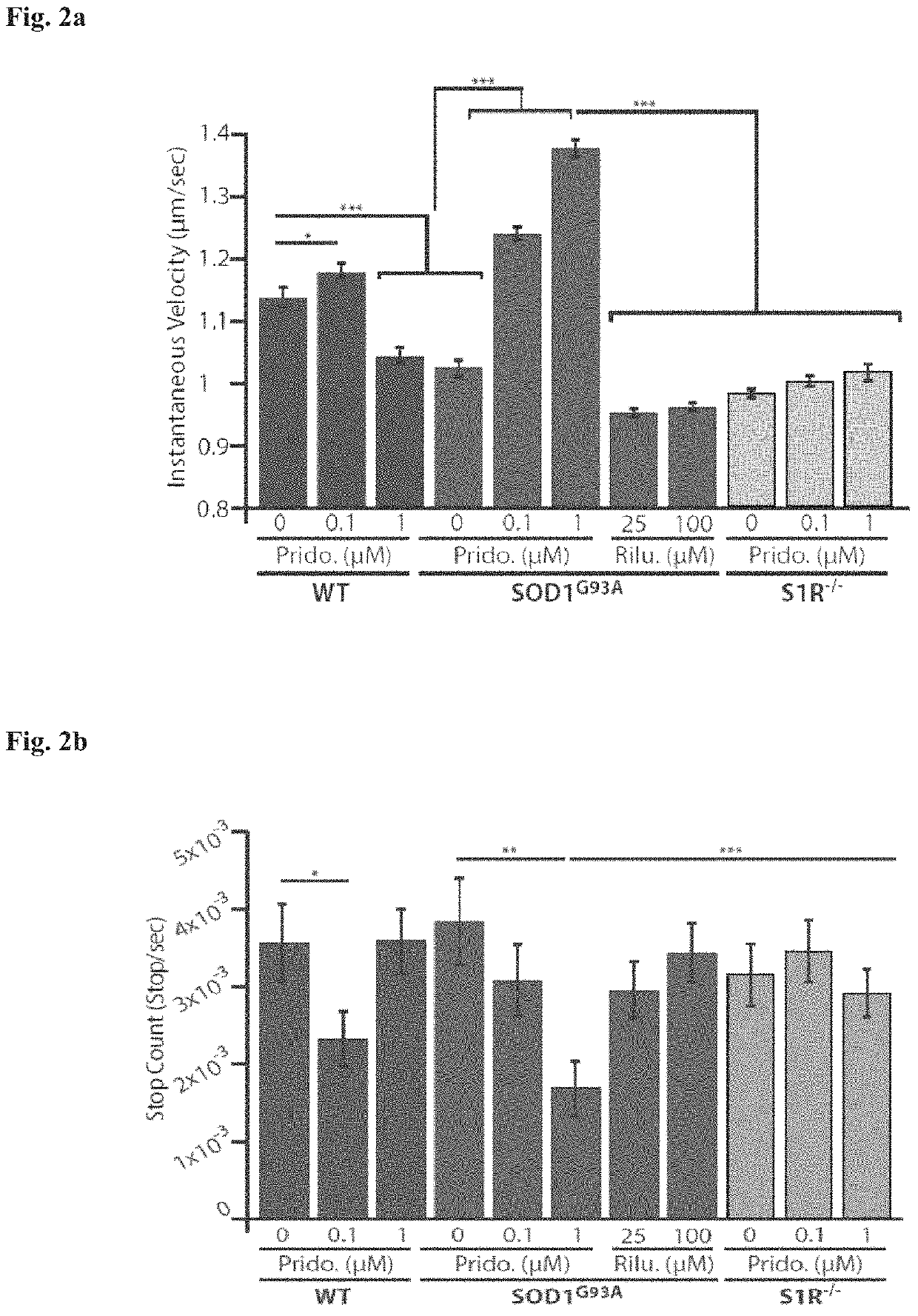
ActiveUS20200179355A1Improves axonal transport deficitEnhances ERK activationNervous disorderPharmaceutical delivery mechanismCholic acidTauroursodesoxycholic acid
Provided herein is a method for treating a human subject afflicted with ALS by administering to the subject a therapeutically effective amount of pridopidine as monotherapy or together with riluzole, edaravone, combination of dextromethorphan / quinidine, sodium phenylbutyrate (PB), tauroursodeoxycholic acid or combination of sodium phenylbutyrate (PB) / tauroursodeoxycholic acid (i.e. AMX0035) as combination or add-on therapy.
Owner:PRILENIA NEUROTHERAPEUTICS LTD
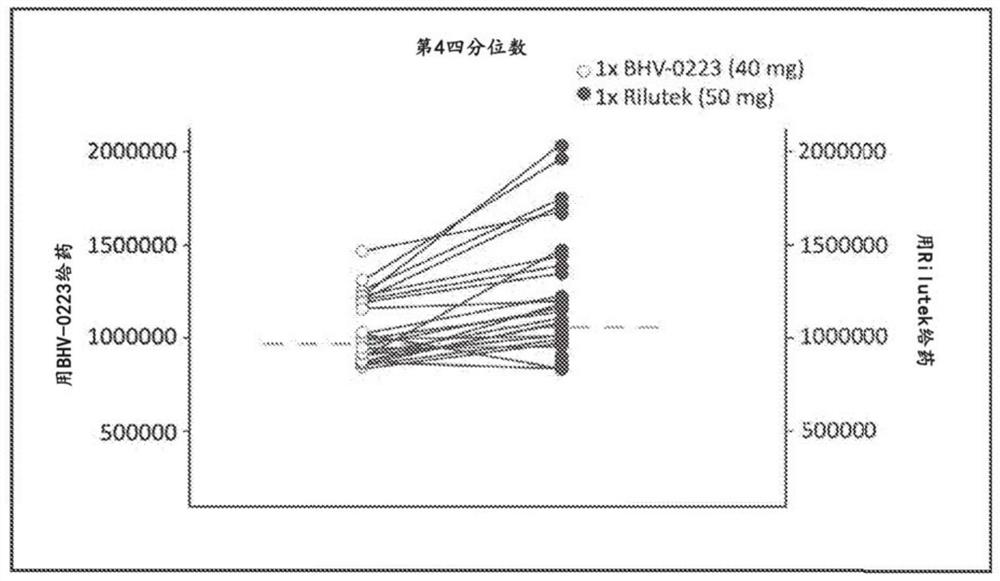
Use of riluzole oral disintigrating tablets for treating diseases
Disclosed are methods of treating a disease in a patient in need thereof, comprising administering to the patient a pharmaceutical composition comprising a therapeutically effective amount of riluzole, or a pharmaceutically acceptable salt thereof, in the form of an oral solid molded fast-dispersing dosage form. Pharmaceutical compositions and kits are also disclosed.
Owner:BIOHAVEN PHARMA HLDG CO LTD
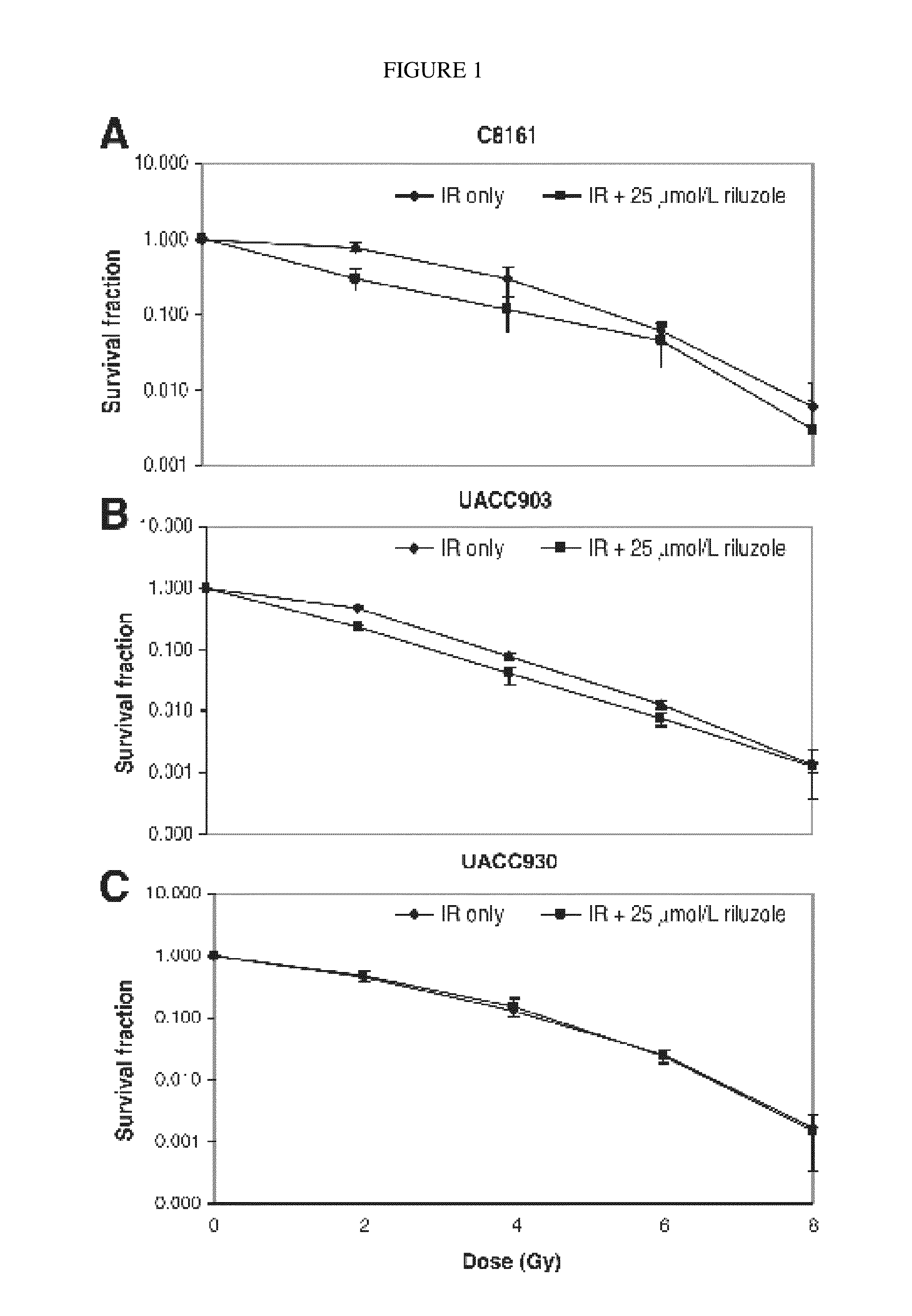
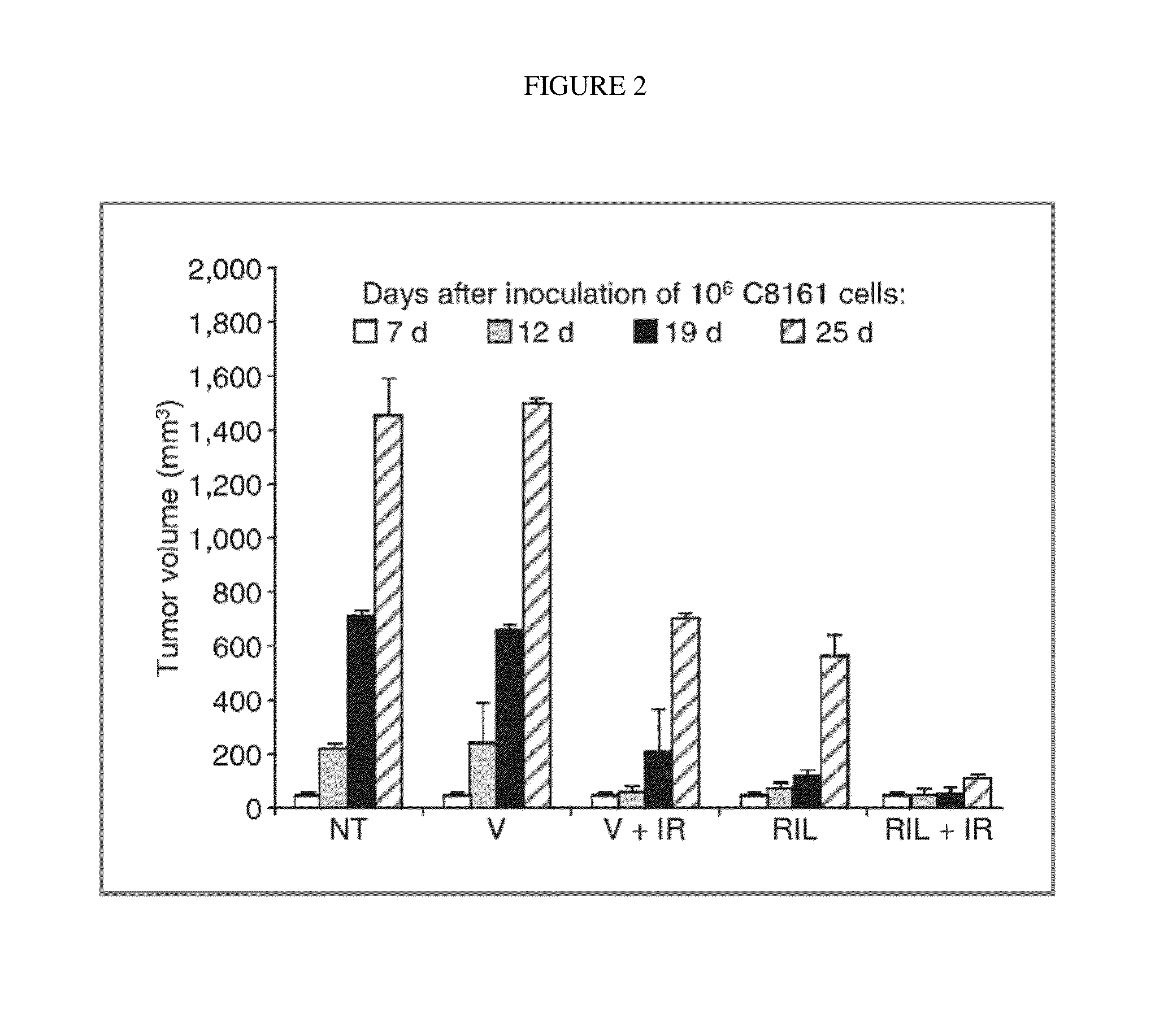
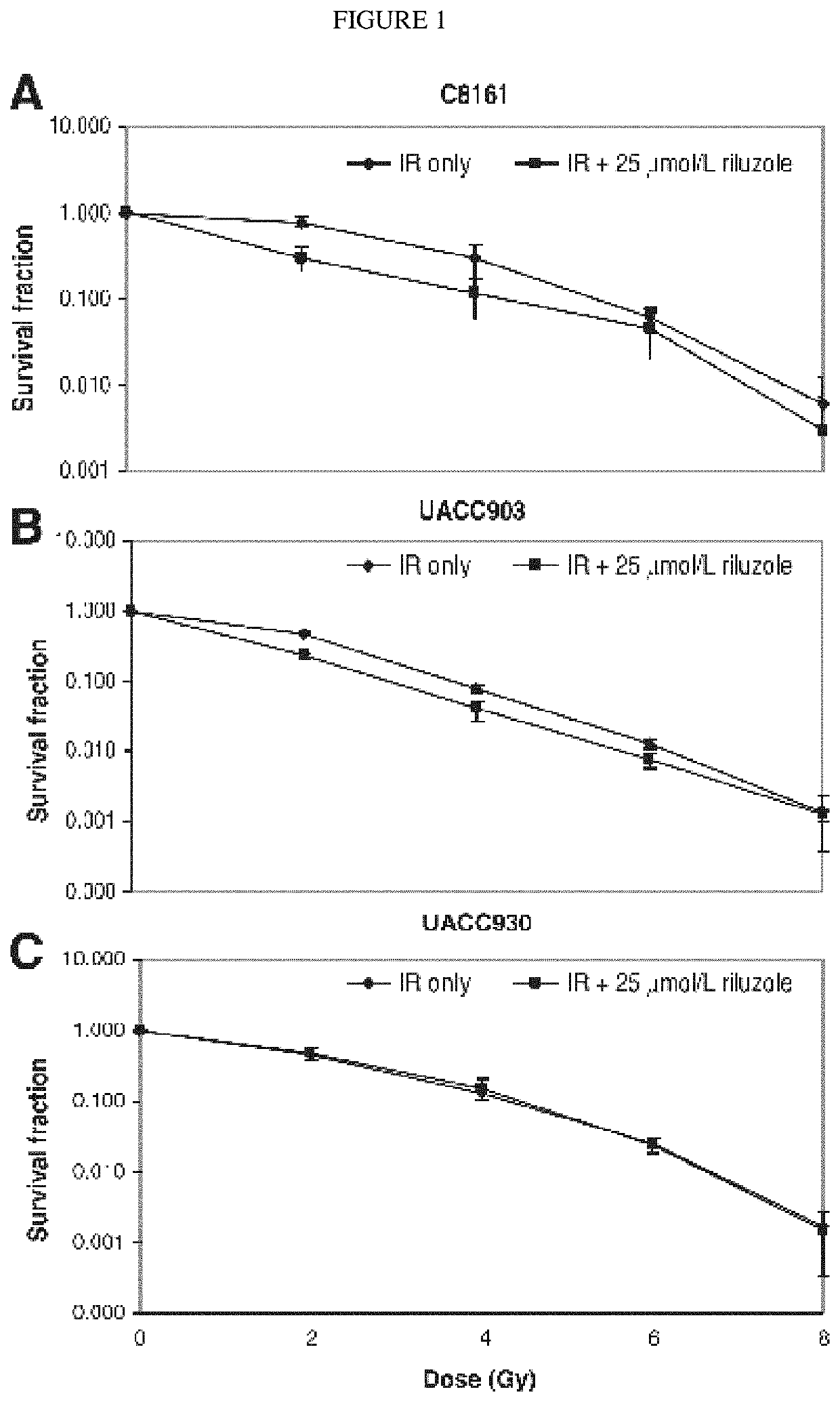
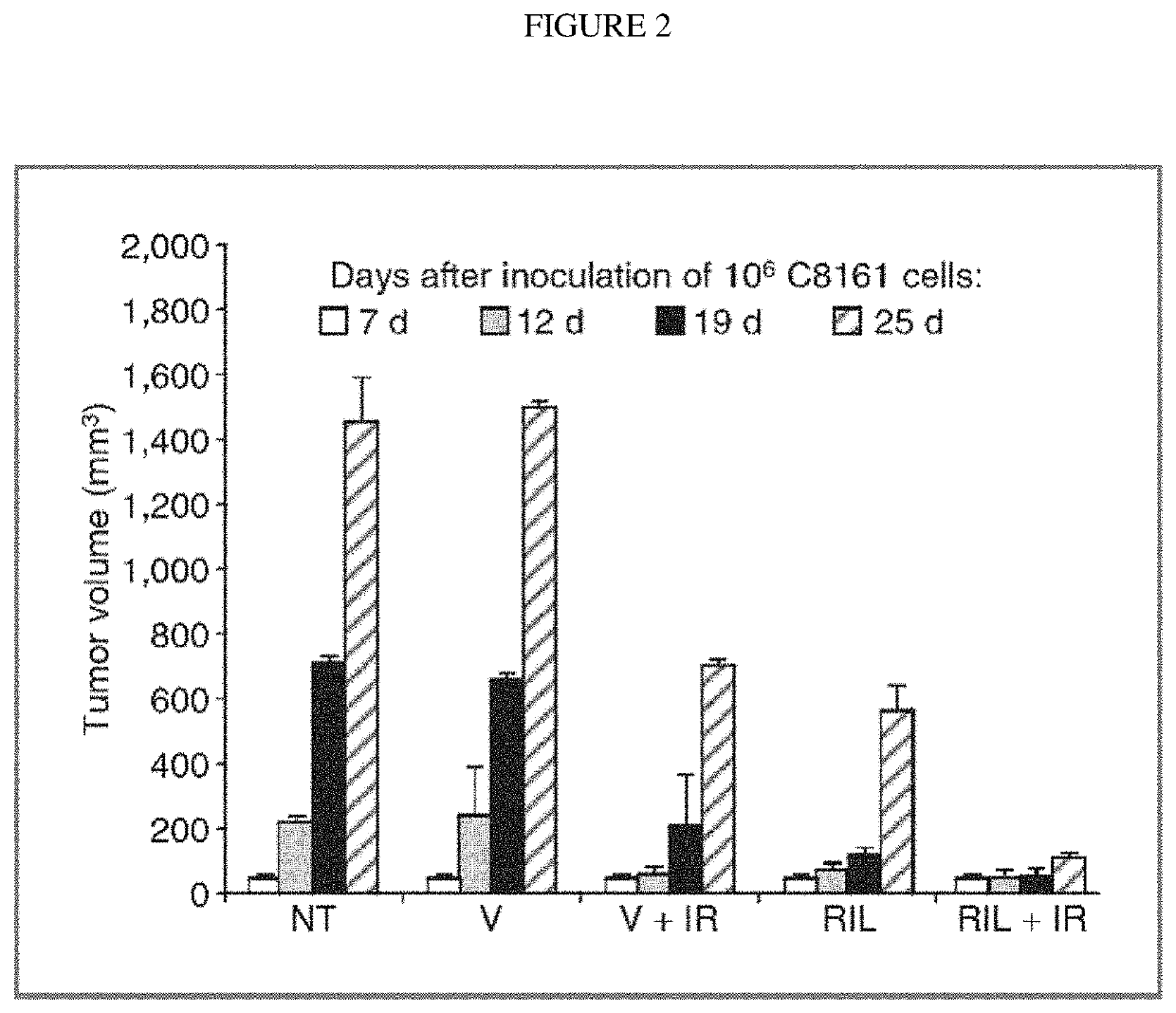
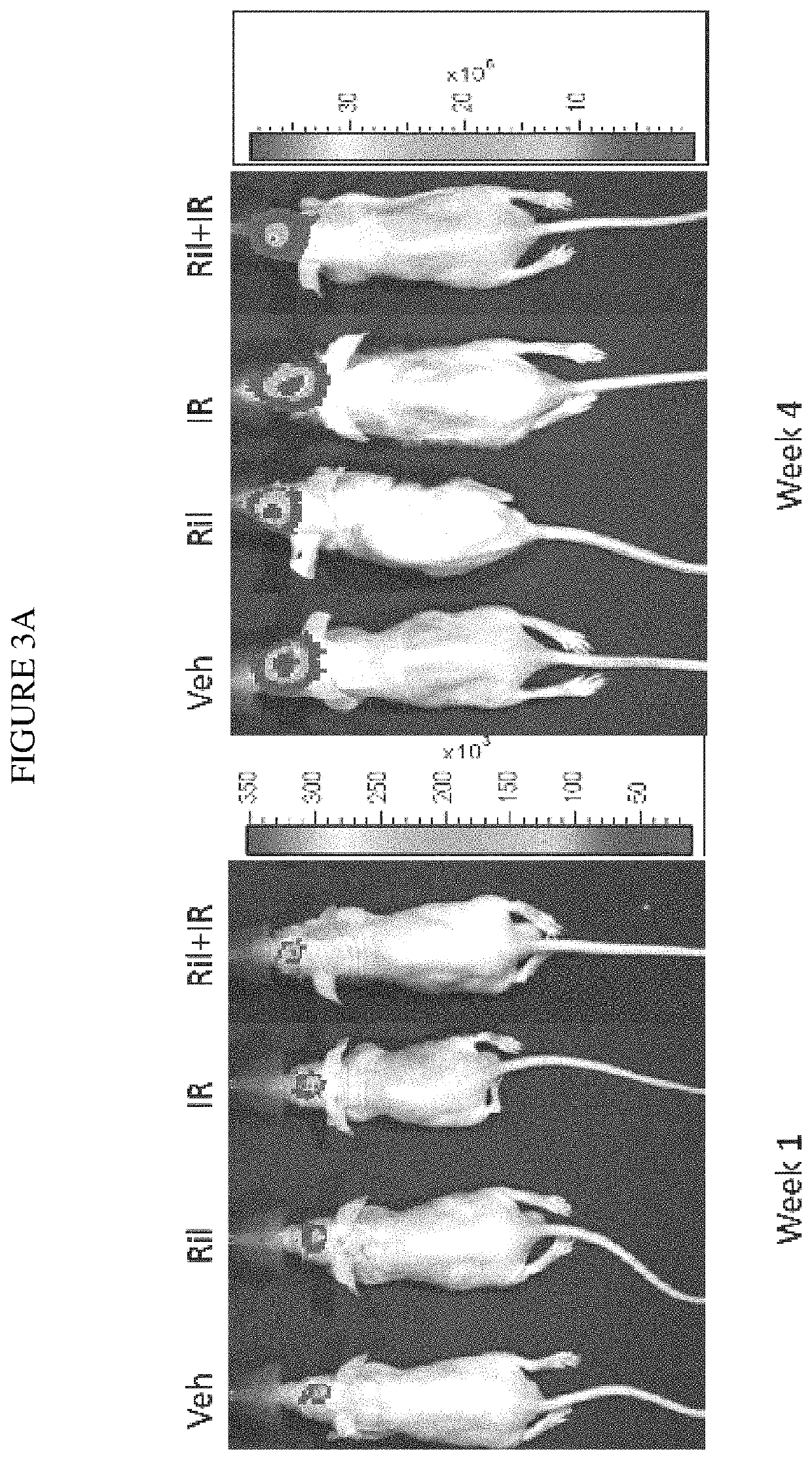
Combination therapy using riluzole to enhance tumor sensitivity to ionizing radiation
ActiveUS20130210872A1Increased radiation sensitivityHigh sensitivityOrganic active ingredientsBiocideWilms' tumorCombination therapy
Disclosed is a method of treating a tumor in a patient, comprising (a) administering riluzole in an amount effective to sensitize the tumor cells to ionizing radiation, and (b) irradiating the tumor cells with ionizing radiation in a dose effective to reduce tumor cell growth. The method can further comprise administering an effective amount of one or more additional therapeutic agents.
Owner:RUTGERS THE STATE UNIV
Method of treating amyotrophic lateral sclerosis with pridopidine
ActiveUS11406625B2Easy to transportIncreased activationNervous disorderMuscular disorderCholic acidTauroursodesoxycholic acid
Owner:PRILENIA NEUROTHERAPEUTICS LTD
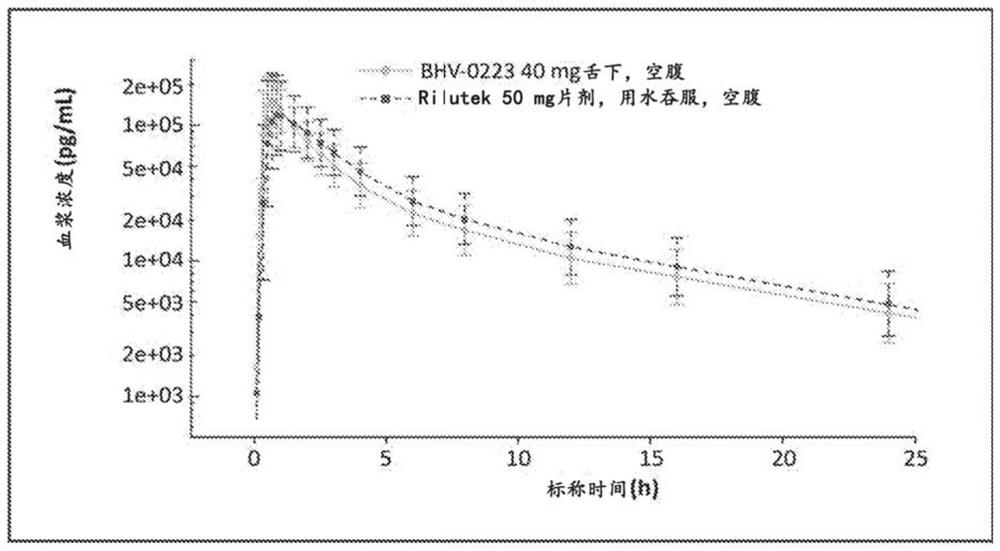
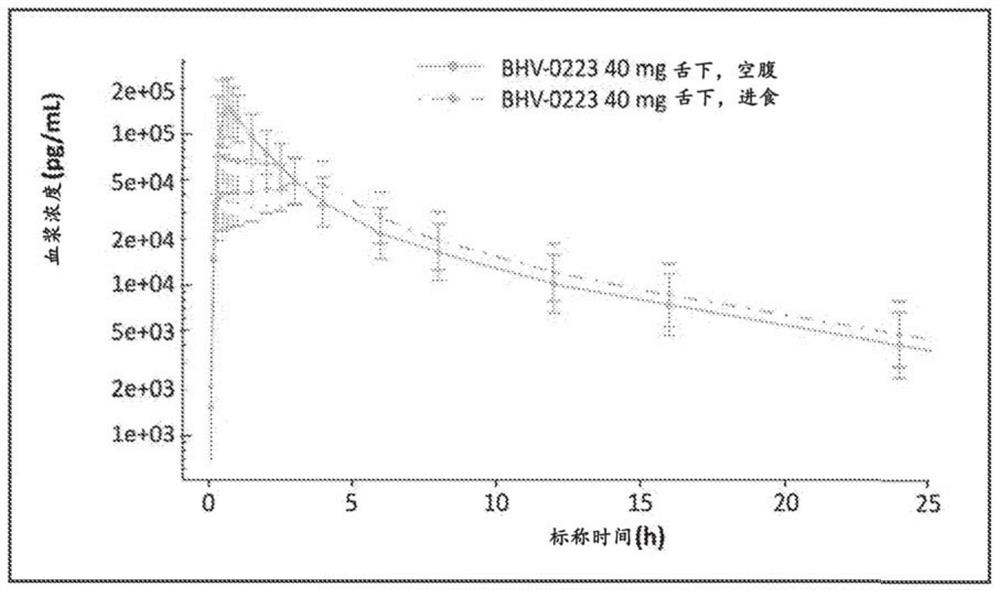
Sublingual administration of riluzole
InactiveCN107735077AOrganic active ingredientsNervous disorderSublingual administrationEndocrinology
Disclosed is sublingual administration of riluzole. In particular, a method for treating a neuropsychiatric disorder or symptom by administering a sublingual formulation of riluzole is provided. In addition, a method of relieving or reducing oral pain using the sublingual formulation of riluzole is disclosed.
Owner:BIOHAVEN PHARMA HLDG CO LTD
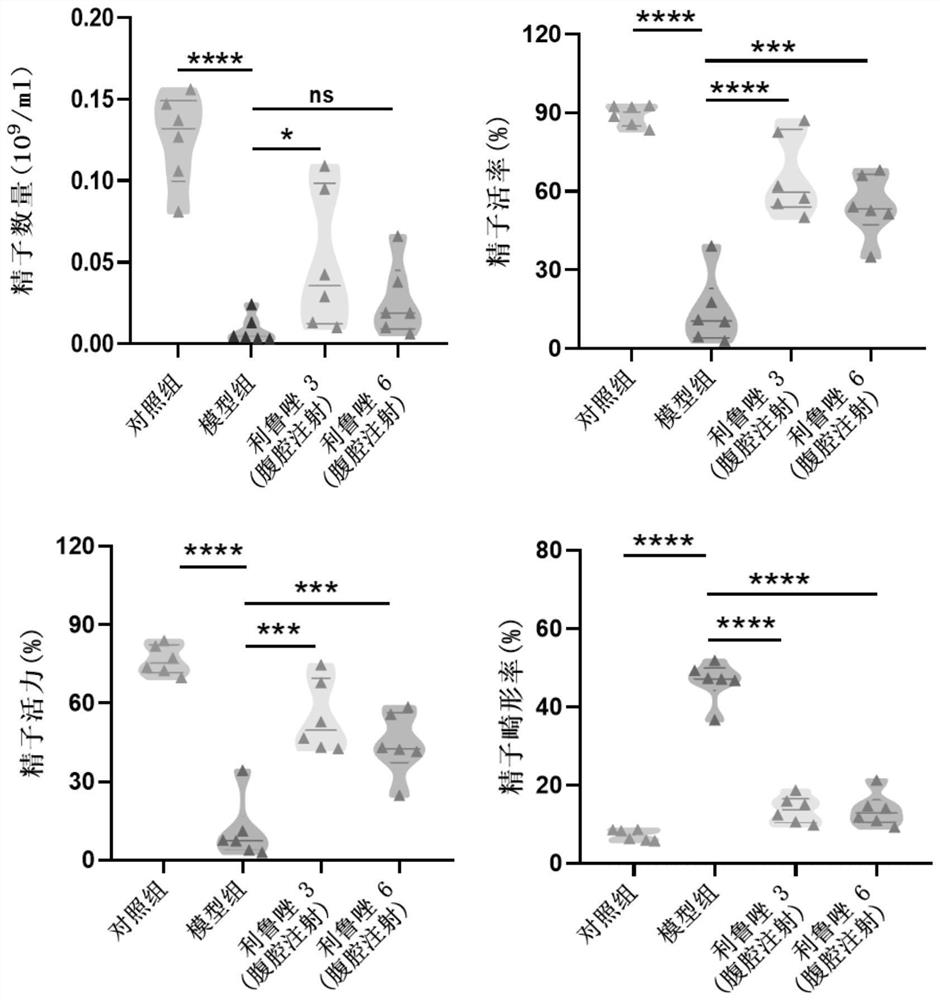
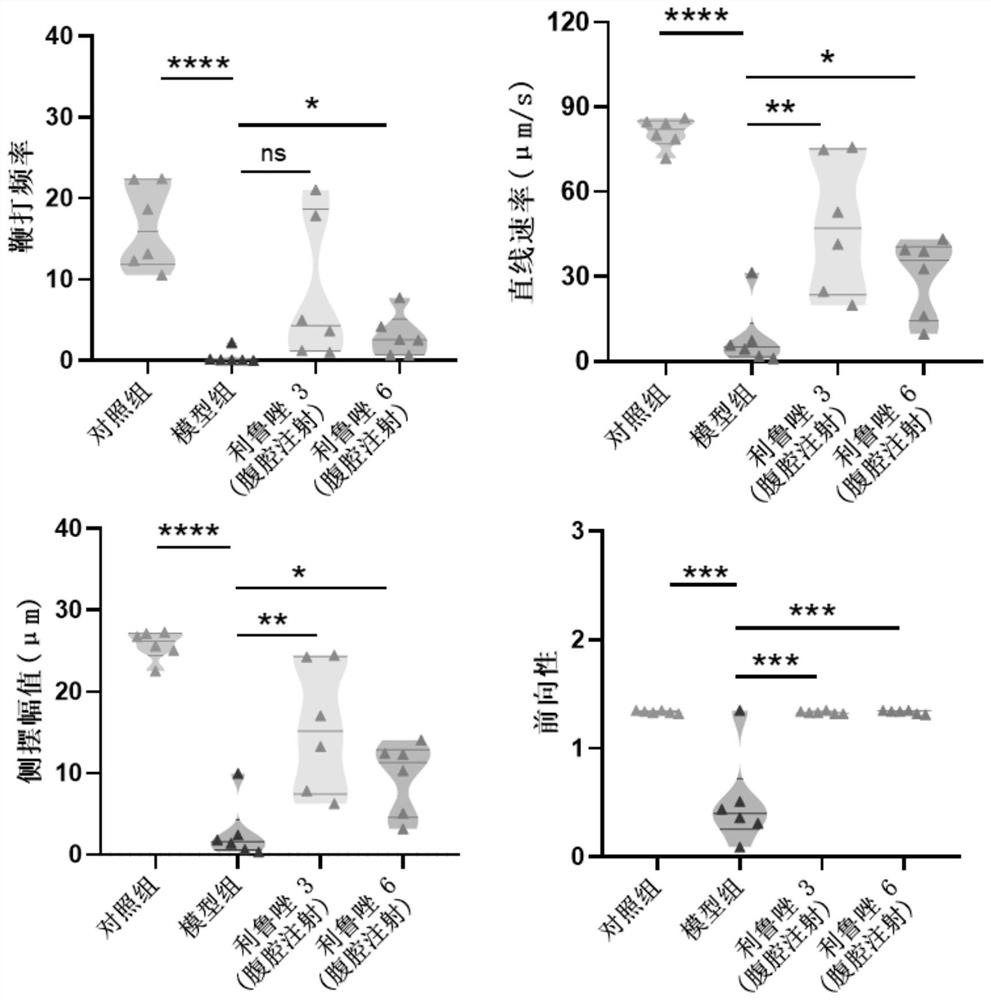
Application of riluzole in treatment of oligospermia
PendingCN114344297ALittle side effectsQuick effectOrganic active ingredientsSexual disorderINFERTILITY MALEPharmacology
The invention provides application of a non-hormone compound riluzole in treatment of oligospermia, provides beneficial help for solving the problem of male infertility, and has potential application prospects in the field of medicines.
Owner:JINAN UNIVERSITY
Prodrugs of riluzole and their method of use
PendingUS20210236470A1Increasing serotonin activityOrganic active ingredientsNervous disorderMelanomaProstate cancer
Pharmaceutical compositions of the invention include substituted riluzole prodrugs useful for the treatment of cancers including melanoma, breast cancer, brain cancer, and prostate cancer through the release of riluzole. Prodrugs of riluzole have enhanced stability to hepatic metabolism and are delivered into systemic circulation by oral administration, and then cleaved to release riluzole in the plasma via either an enzymatic or general biophysical release process.
Owner:BIOHAVEN THERAPEUTICS LTD
Use of riluzole for the treatment of multiple sclerosis
InactiveUS20050171168A1Improve bioavailabilityOrganic active ingredientsBiocideMS multiple sclerosisPharmacology
Methods and compositions for the treatment of multiple sclerosis comprising riluzole (6-(trifluoromethoxy)-benzothiazolamine) are disclosed herein.
Owner:VER VOOR CHRISTELIJK WETENSHAPPELIKJK ONDERWIJS
Combination therapy using riluzole to enhance tumor sensitivity to ionizing radiation
ActiveUS10864271B2High sensitivityEnhanced radiationRadiosensitized materialsTumor reductionOncology
Owner:RUTGERS THE STATE UNIV
A kind of riluzole orally disintegrating tablet and preparation method thereof
ActiveCN111821289BMeet the quality standard requirementsImprove quality stabilityOrganic active ingredientsAntineoplastic agentsSodium bicarbonateMedicine
The invention relates to a riluzole orally disintegrating tablet and a preparation method thereof, belonging to the field of preparations. The tablet is prepared from riluzole, sodium bicarbonate, filler, disintegrant, lubricant and flavoring agent. The preparation method is as follows: 1) Weigh the prescribed amount of riluzole, sodium bicarbonate, filler and 50% of the prescribed amount of disintegrant, mix evenly, and prepare the mixture for later use; 2) add the mixture in step 1 to granulate with ethanol aqueous solution, and dry After sieving, the dry granules are ready for use; 3) Add 50% of the disintegrating agent of the prescribed amount and the flavoring agent of the prescribed amount to the dry granules of step 2, mix evenly, then add the lubricant of the prescribed amount, mix evenly, and compress to obtain Riluzole orally disintegrating tablet. The orally disintegrating tablet prepared by the invention has short disintegration time limit, high dissolution rate and no gritty feeling, meets the quality standard requirements of the orally disintegrating tablet, and is suitable for industrial scale-up production.
Owner:LUNAN PHARMA GROUP CORPORATION
Oral film compositions and dosage forms having precise active dissolution profiles
An oral film in an individual unit dose for delivery of one or more actives is disclosed herein, the film having a precisely calculated and controlled active dissolution profile. A wide variety of actives may be used, including, for example, clobazam, diazepam, or riluzole. Also disclosed are methods of treating a variety of diseases and conditions, for example., epilepsy and seizures, by administering the oral film disclosed herein.
Owner:阿奎蒂夫疗法公司
Use of riluzole oral disintigrating tablets for treating diseases
PendingUS20210315865A1Enhance memoryOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
Disclosed are methods of treating a disease in a patient in need thereof, comprising administering to the patient a pharmaceutical composition comprising a therapeutically effective amount of riluzole, or a pharmaceutically acceptable salt or prodrug thereof, in the form of an oral solid molded fast-dispersing dosage form. Pharmaceutical compositions and kits are also disclosed.
Owner:BIOHAVEN THERAPEUTICS LTD
Combination therapy with glatiramer acetate and riluzole
The present invention provides a method for providing neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection, which comprises periodically administering to the subject a certain amount of glatiramer acetate and a certain amount of 2-amino-6 - Trifluoromethoxybenzothiazole, wherein said amount is effective to provide neuroprotection to said subject's central or peripheral nervous system when taken together. The present invention also provides a package comprising glatiramer acetate, 2-amino-6-trifluoromethoxybenzothiazole, and a composition for use together to provide neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection. Neuroprotective Instructions for Use. Furthermore, the present invention provides a pharmaceutical composition comprising an amount of glatiramer acetate and an amount of 2-amino-6-trifluoromethoxybenzothiazole, wherein said amounts are effective to provide for Neuroprotection of the subject's central or peripheral nervous system. The present invention also provides a pharmaceutical combination comprising a certain amount of glatiramer acetate and a certain amount of 2-amino-6-trifluoromethoxybenzothiazole in separate dosage forms, the combination is effective for providing Neuroprotection of the central or peripheral nervous system. Additionally, the combination therapy may be used to treat a subject afflicted with multiple sclerosis or a subject afflicted with amyotrophic lateral sclerosis.
Owner:TEVA PHARMA IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com