Riluzole orally disintegrating tablet and preparation method thereof
A technology of riluzole mouth and riluzole, which is applied in the field of riluzole orally disintegrating tablets and its preparation, can solve the problem that there is no mention of the dissolution rate of riluzole emulsion and the stability of riluzole tablets. Solve problems such as increased dissolution of riluzole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
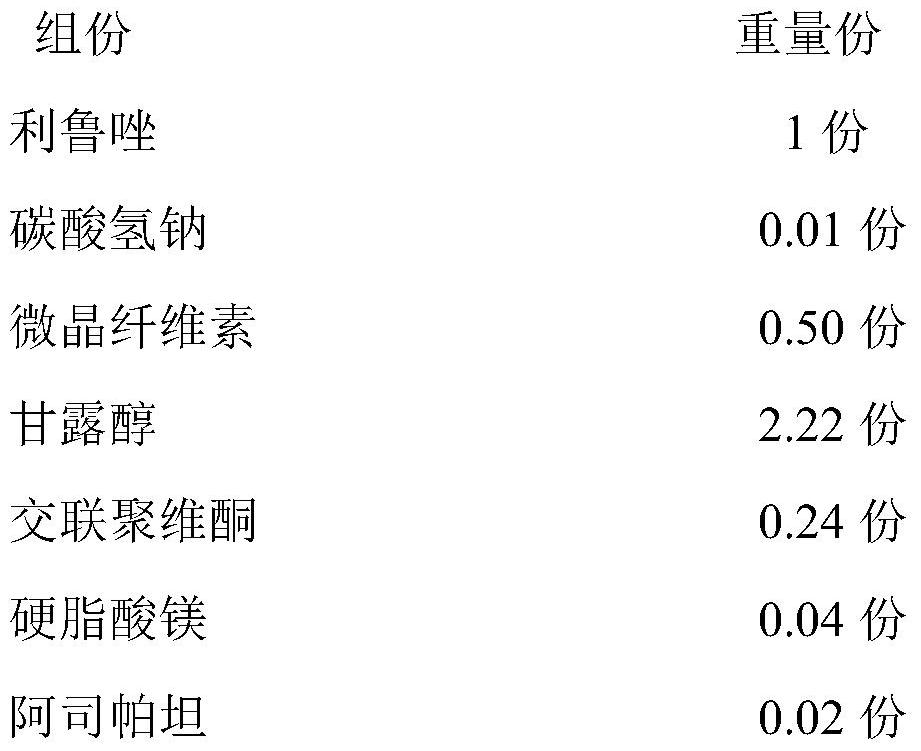
[0034] 1) Prescription
[0035]
[0036] 2) Preparation method
[0037] 1) Weigh the riluzole, sodium bicarbonate, microcrystalline cellulose, mannitol and 50% of the prescription amount of crospovidone and mix evenly, and the mixture is set aside;
[0038] 2) adding the mixture in step 1) into an aqueous ethanol solution with a mass concentration of 10% to 50% to granulate, passing through a 60-mesh sieve after drying, and dry the granules for later use;
[0039] 3) Add 50% of the prescribed amount of crospovidone and the prescribed amount of aspartame to the dry granules in step 2), mix well, then add the prescribed amount of magnesium stearate, mix evenly, and press to obtain Rilu Azole orally disintegrating tablets.
Embodiment 2
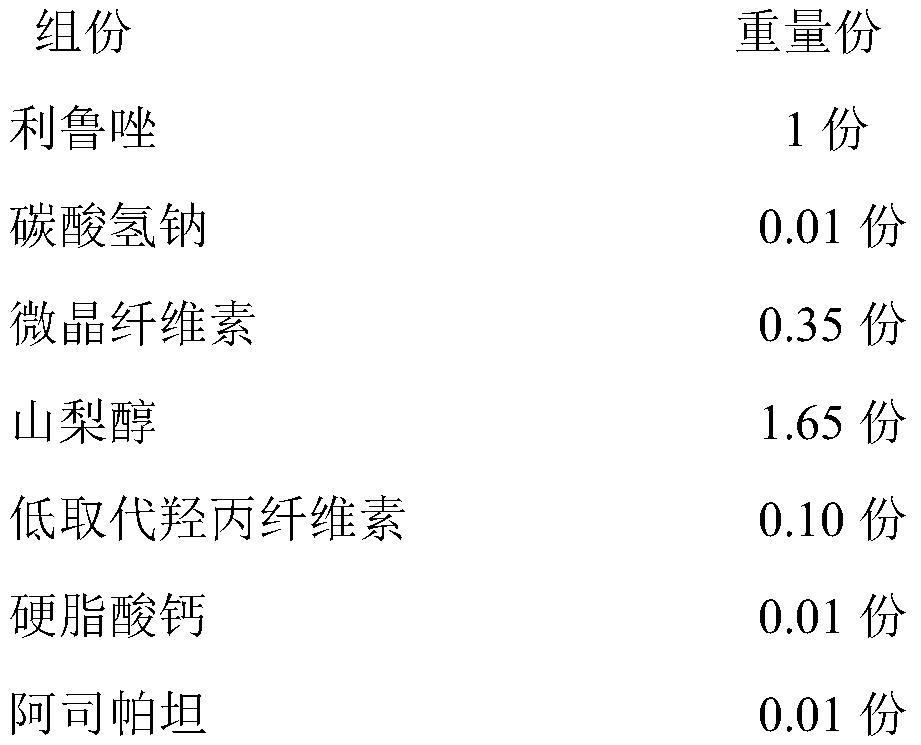
[0041] 1) Prescription
[0042]
[0043] 2) Preparation method
[0044] 1) Weigh the prescription amount of riluzole, sodium bicarbonate, microcrystalline cellulose, sorbitol and 50% of the prescription amount of low-substituted hydroxypropyl cellulose, mix well and set aside;
[0045] 2) adding the mixture in step 1) into an aqueous ethanol solution with a mass concentration of 10% to 50% to granulate, passing through a 60-mesh sieve after drying, and dry the granules for later use;
[0046] 3) Add 50% prescription amount of low-substituted hydroxypropyl cellulose and the prescription amount of aspartame to the dry granules in step 2), mix uniformly, then add the prescription amount of calcium stearate, mix uniformly, and compress to obtain a benefit Luzole orally disintegrating tablets.
Embodiment 3
[0048] 1) Prescription
[0049]
[0050]
[0051] 2) Preparation method
[0052] 1) Weigh the crospovidone, riluzole, sodium bicarbonate, microcrystalline cellulose, mannitol and 50% prescription quantity of the prescription, mix well and set aside;
[0053] 2) adding the mixture in step 1) into an aqueous ethanol solution with a mass concentration of 10% to 50% to granulate, passing through a 60-mesh sieve after drying, and dry the granules for later use;
[0054] 3) Add 50% of the prescription amount of crospovidone and the prescription amount of acesulfame-K to the dry granules in step 2), mix evenly, then add the prescription amount of sodium stearyl fumarate, mix evenly, and press to obtain the benefit Luzole orally disintegrating tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com