Riluzole sustained-release oral-administration suspension
A technology of oral suspension and riluzole, which is applied in the directions of liquid delivery, emulsion delivery, and drug combination, etc., can solve the problems of poor compliance of administration and too frequent administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
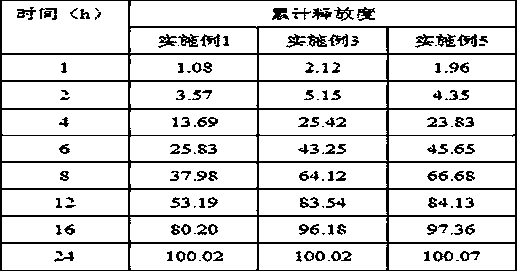
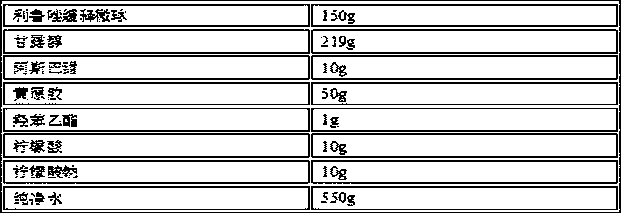
[0025] prescription composition
[0026]
[0027] Preparation Process:
[0028] (1) Dissolving riluzole in ethyl cellulose aqueous dispersion, wherein the solid content ratio of riluzole to ethyl cellulose aqueous dispersion is 1:3, and spray drying to prepare ethyl cellulose microspheres;
[0029] (2) Weigh the prescription amount of xylitol, acesulfame potassium, citric acid and sodium citrate and dissolve in a certain amount of solvent to obtain a solution containing diluent, flavoring agent and buffer;
[0030] (3) Weigh the prescribed amount of xanthan gum, sodium benzoate and riluzole sustained-release microspheres, and disperse them in a certain amount of solvent to obtain a suspension containing suspending agents, preservatives and active ingredients;
[0031] (4) Mix the above solution with the suspension and stir thoroughly, and dilute to the mark with the remaining solvent.
Embodiment 2
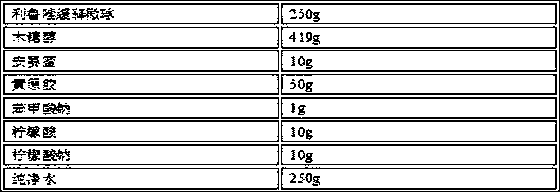
[0033] prescription composition
[0034]
[0035] Preparation Process:
[0036] (1) Dissolving riluzole in ethyl cellulose aqueous dispersion, wherein the solid content ratio of riluzole to ethyl cellulose aqueous dispersion is 1:1, and spray drying to prepare ethyl cellulose microspheres;
[0037] (2) Dissolve the prescription amount of xylitol, sucralose, citric acid and sodium citrate in a certain amount of solvent to obtain a solution containing diluent, flavoring agent and buffer;
[0038] (3) Weigh the prescribed amount of sodium carboxymethylcellulose, sodium benzoate and riluzole sustained-release microspheres, and disperse them in a certain amount of solvent to obtain a suspension containing suspending agents, preservatives and active ingredients;
[0039] (4) Mix the above solution with the suspension and stir thoroughly, and dilute to the mark with the remaining solvent.
Embodiment 3
[0041]
[0042] Preparation Process:
[0043] (1) Dissolving riluzole in ethyl cellulose aqueous dispersion, wherein the solid content ratio of riluzole to ethyl cellulose aqueous dispersion is 1:2, and spray drying to prepare ethyl cellulose microspheres;
[0044] (2) Dissolve the prescription amount of mannitol, aspartame, citric acid and sodium citrate in a certain amount of solvent to obtain a solution containing diluent, flavoring agent and buffer;
[0045] (3) Weigh the prescribed amount of xanthan gum, ethylparaben and riluzole sustained-release microspheres, and disperse them in a certain amount of solvent to obtain a suspension containing suspending agents, preservatives and active ingredients;
[0046] (4) Mix the above solution with the suspension and stir thoroughly, and dilute to the mark with the remaining solvent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com