Use of Leonurine in the Preparation of Retinal Optic Nerve Protective Drugs
A technology of leonurine and retina, which is applied to the preparation of drugs for the treatment of retinal vascular diseases, optic nerve inflammation and degenerative diseases, and optic nerve protective drugs. In the field of retina preparation, leonurine can solve the problems of treating eye diseases that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
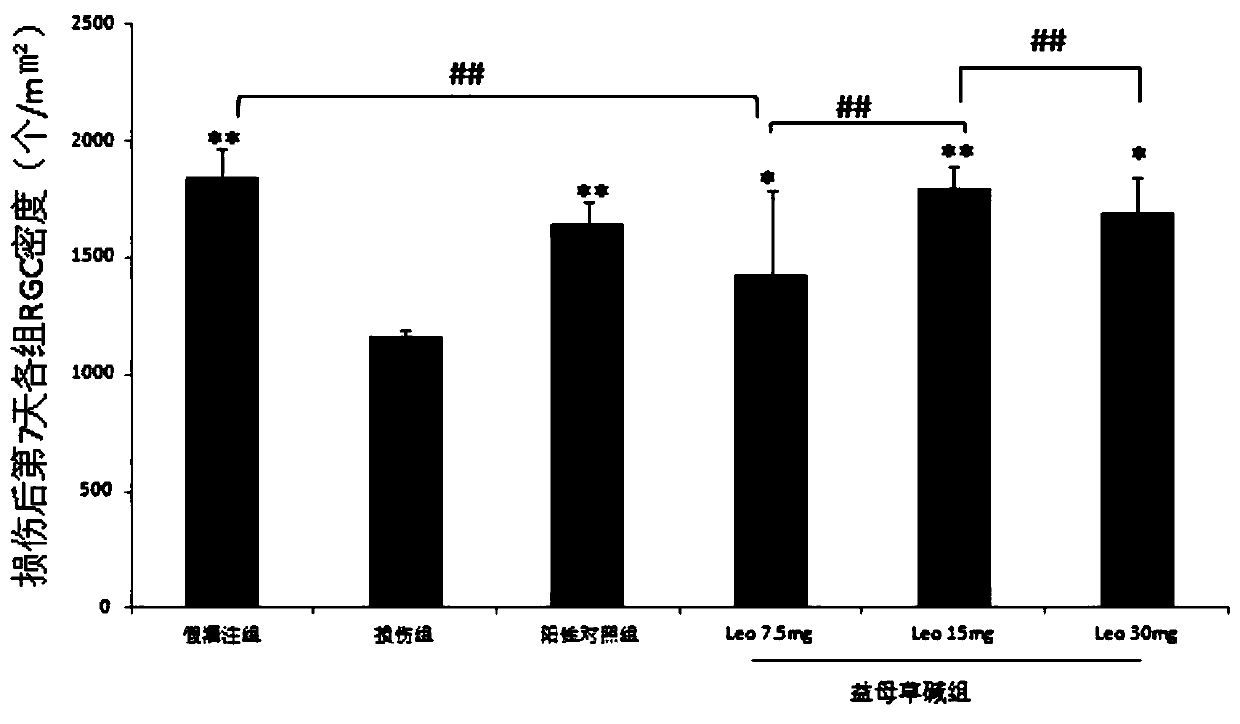
[0044] Example 1: Leonurine improves the survival rate of damaged RGCs
[0045] (1) Animal model establishment
[0046] a. Intraperitoneal injection 1 hour before modeling. The positive control group received 100 mg / kg of Ginkgo biloba extract, three dosage groups of Leonurine component, 7.5 mg / kg, 15 mg / kg, and 30 mg / kg, respectively, and the injury group received the same volume of normal saline;
[0047] b. Intramuscular injection of ketamine (80mg / kg) and xylazine (12mg / kg). After general anesthesia of the animal, the area around the eyes was cleaned and disinfected. Lincomycin eye drops flush the eyes;
[0048] c. Under the ophthalmic operating microscope, anterior chamber perfusion was performed on the injury group, the positive control group and the motherwort group. Use a 4.5mm scalp vein needle connected to a 0.9% normal saline bag to perform anterior chamber puncture, avoid blood vessels at 3 o'clock and 9 o'clock, lift the saline bag to a height of 150 cm from th...
Embodiment 2
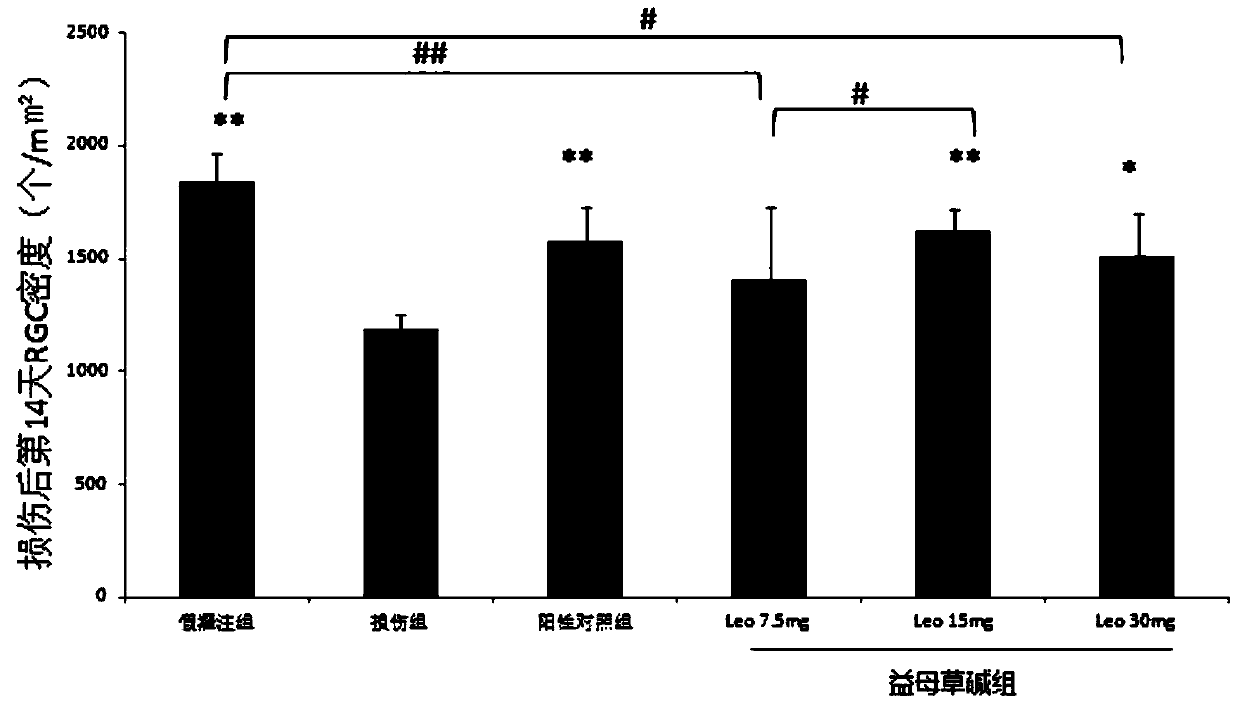
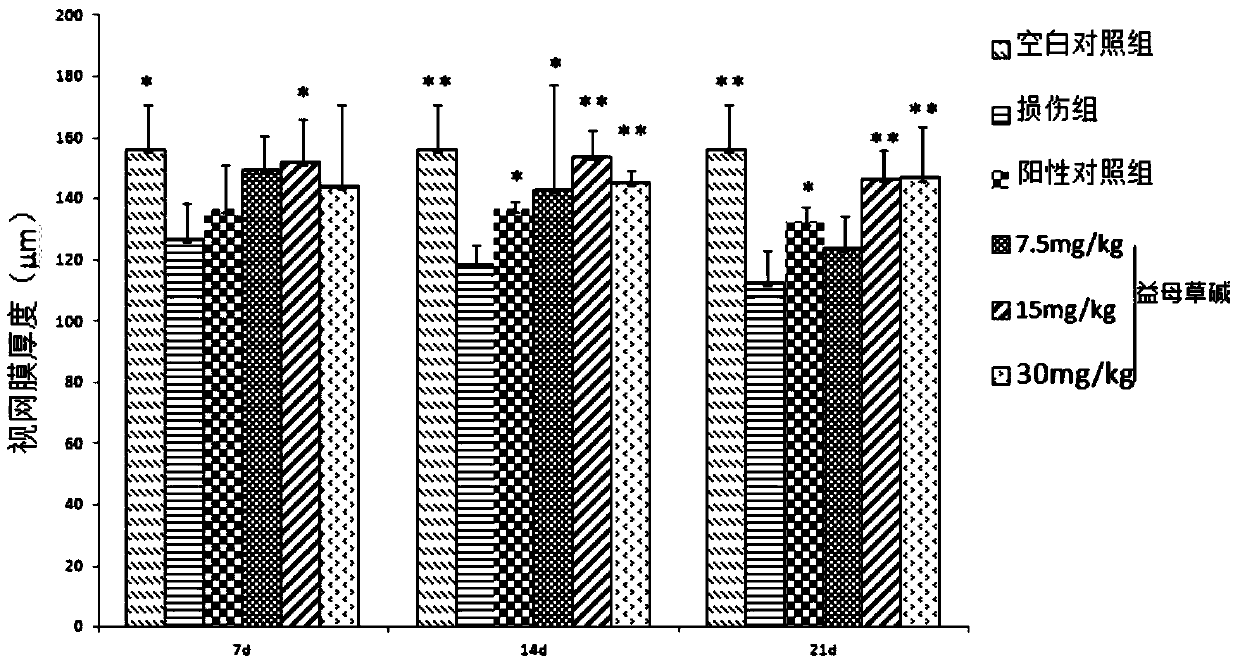
[0063] Example 2: Leonurine reduces full-thickness retinal damage after ischemia-reperfusion injury
[0064] (1) each group is processed by the method in embodiment 1;
[0065] (2) Making paraffin sections
[0066] a. Rats were killed on the 7th, 14th, and 21st days after modeling, and the eyeballs were fixed in the eyeball fixation solution for 48 hours and rinsed for 2 hours;
[0067] b. Dehydration: 50% alcohol, 70% alcohol, 85% alcohol, 95% alcohol, 100% alcohol, 100% alcohol for 2 hours each;
[0068] c. Transparent: transfer to 1 / 2 xylene + absolute ethanol for 2 hours, transfer to pure xylene for 1.5 hours, and pure xylene for 1.5 hours;
[0069] d. Wax immersion: place the treated specimen in 1 / 2 powdered paraffin + 1 / 2 xylene, and place it in a 40°C incubator overnight;
[0070] e. Embedding: raise the temperature of the incubator to 60°C, lift and store the wax 3 times, each time for 1-2 hours, pour the material into the mold on the ironing board, and then put it ...
Embodiment 3
[0083] Example 3: Leonurine reduces the apoptosis rate of retinal ganglion cells after ischemia-reperfusion injury
[0084] (1) each group is processed by the method of embodiment 1;
[0085] (2) Frozen section production
[0086] a. On the 1st and 3rd day after modeling respectively, after deeply anesthetizing the rats with chloral hydrate, use 200ml of filtered 4% paraformaldehyde, fix the eyeballs by cardiac perfusion, and soak them in 4% paraformaldehyde Fixed for 2 hours;
[0087] b. Dehydration in 10%, 20%, 30% sucrose successively;
[0088] c. After dehydration, cut off the cornea at 1mm of the limbus, remove the lens and vitreous tissue, absorb the liquid in the eye cup, embed it in the mold with OCT embedding agent, and then place it in a -20°C refrigerator until it is formed. Transfer to -80°C refrigerator and freeze for 1 hour;
[0089] d. Use a cryostat to slice along the sagittal plane of the eyeball, the thickness of the slice is 10 μm, stick it on a slide-pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com