Prevention and treatment of retinal ischemia and edema
a retinal ischemia and edema technology, applied in the field of retinal ischemia and edema prevention and treatment, can solve the problems of retinal ischemia and/or retinal edema reduction, and achieve the effects of inhibiting leukocyte interaction, inhibiting leukocyte interaction, and inhibiting leukocyte interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Prevention of Leukostasis and Vascular Leakage Diabetic Retinopathy via ICAM-1 Inhibition
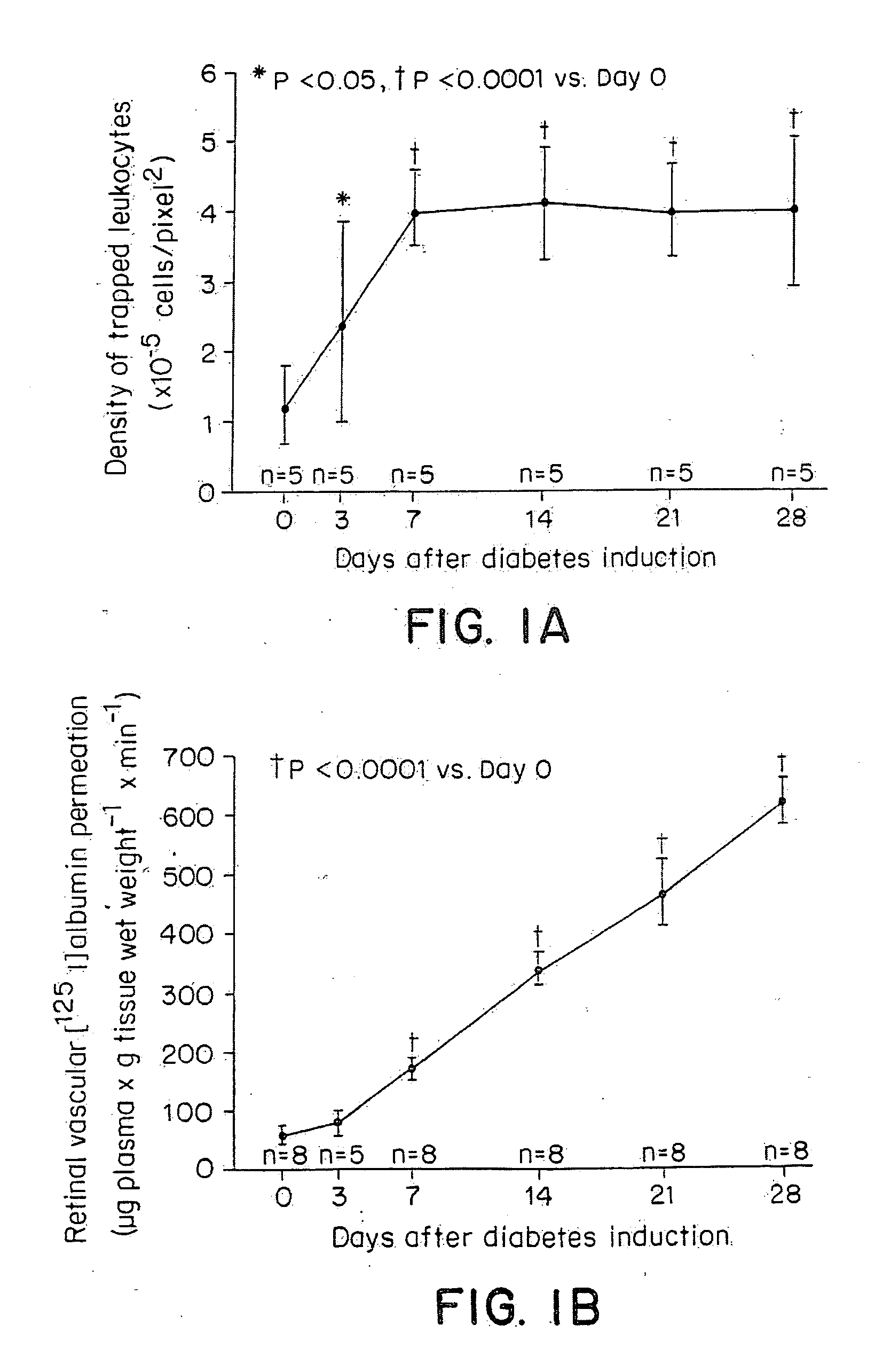
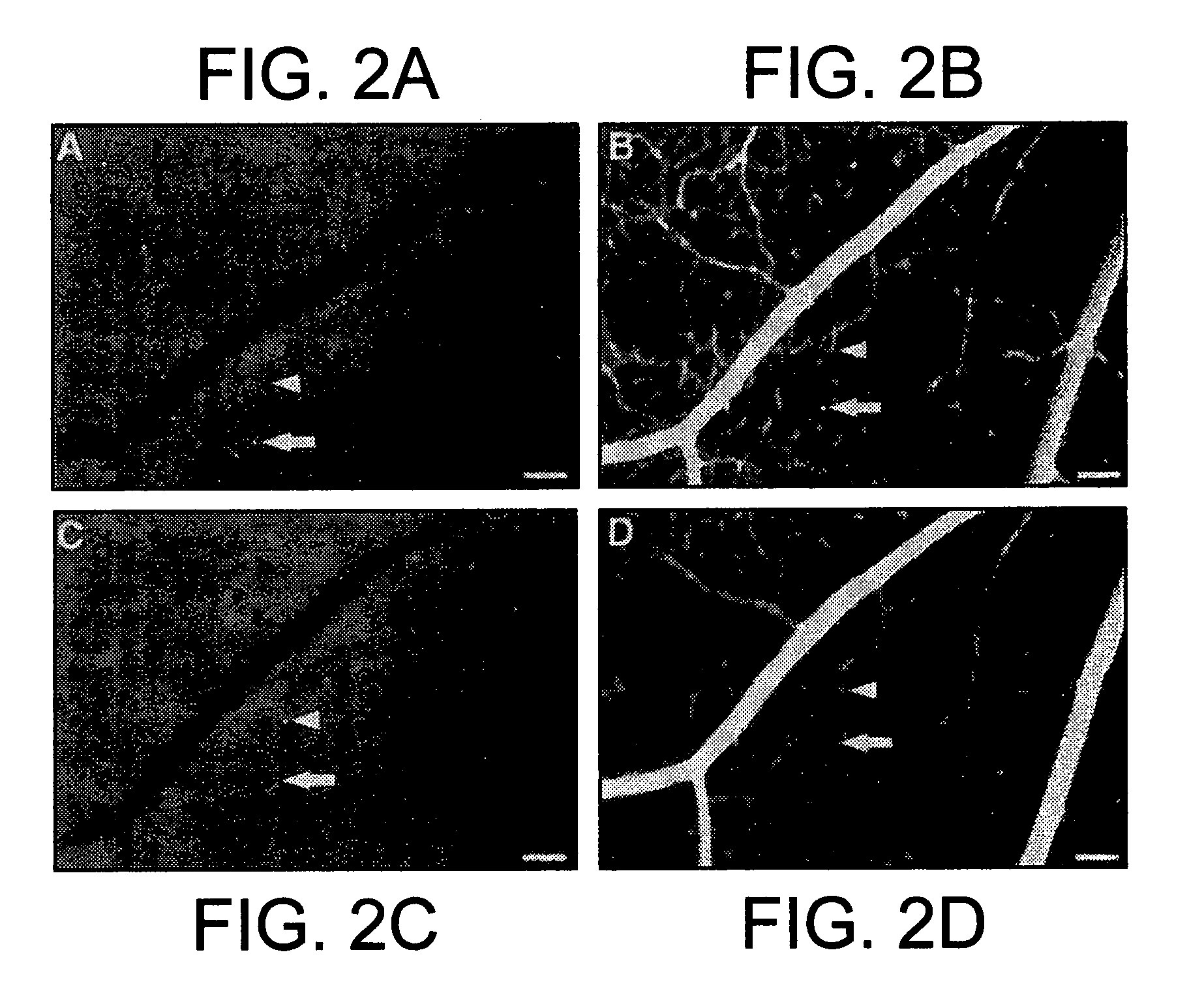
[0091] Diabetic retinopathy is a leading cause of adult vision loss and blindness. Much of the retinal damage that characterizes the disease results from retinal vascular leakage and non-perfusion. This study demonstrates that diabetic retinal vascular leakage and non-perfusion are temporally and spatially associated with retinal leukocyte stasis (leukostasis) in the rat model of streptozotocin-induced diabetes. Retinal leukostasis increases within days of developing diabetes and correlates with the increased expression of retinal intercellular adhesion molecule-1 (ICAM-1). ICAM-1 blockade with a monoclonal antibody prevents diabetic retinal leukostasis (e.g., resulting in ischemia) and vascular leakage (e.g., resulting in edema) by 48.5% and 85.6%, respectively. These data identify the causal role of leukocytes in the pathogenesis of diabetic retinopathy and demonstrate the important utility o...
example 2
Integrin-Mediated Neutrophil Adhesion and Retinal Leukostasis in Diabetes
Introduction:
[0107] Leukocyte-endothelial cell interactions in tissues are mediated by adhesion molecules expressed on the surface of leukocytes and endothelial cells. Immunoglobulin superfamily molecules such as ICAM-1 are expressed on endothelial cells and bind to β2-integrins expressed on leukocytes. The integrins are transmembrane receptors that consist of noncovalently bound heterodimers composed of α- and β-chains. The 2-integrins are operative in leukocyte adhesion and include LFA-1 (lymphocyte function associated antigen, CD11a / CD18), Mac-1 (leukocyte adhesion receptor, CD11b / CD18) and p150 / 95 (CD11c / CD18). Each of the β2-integrins has a common α-chain in combination with a unique α-chain. CD18 is required for the firm attachment of healthy human neutrophils to human umbilical vein endothelial cells.
[0108] In vivo studies from our laboratory have investigated the role of leukocytes in diabetic retin...
example 3
Vascular Endothelial Growth Factor (VEGF)-Induced Retinal Vascular Permeability is Mediated by ICAM-1
SUMMARY
[0130] Two prominent VEGF-induced retinal effects are vascular permeability and capillary non-perfusion. The mechanisms by which these effects occur are not completely known. Using a rat model, it is shown that intravitreous injections of VEGF precipitate an extensive retinal leukocyte stasis (leukostasis) that coincides with enhanced vascular permeability and capillary non-perfusion. The leukostasis is accompanied by the upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in the retina. The inhibition of ICAM-1 bioactivity with a neutralizing antibody prevents the permeability and leukostasis increases by 79% and 54%, respectively. These data are the first to demonstrate that a non-endothelial cell type contributes to VEGF-induced vascular permeability. Additionally, they identify a potential mechanism for VEGF-induced retinal capillary non-perfusion.
[013...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com