Fluorescent quantitative PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof
A technology of mycoplasma pneumoniae and primer pairs, which is applied in the field of pathogen detection, can solve the problems of clinical application limitations, high false positive rate, continuous positive and other problems, and achieve good detection effect and application value, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the acquisition of the fluorescent PCR primer that is used to detect Mycoplasma pneumoniae
[0031] 1. Design and synthesis of primer pairs
[0032] According to the genome DNA sequence (GenBank sequence number: CP002077) of the human Mycoplasma pneumoniae international standard strain FH (ATCC15531) published on GenBank, the 16S rRNA (GenBank sequence number: AF132740) and ATPase gene sequence (GenBank sequence number: AF132740) of the strain (GenBank The sequence number: U43738) was used as a template, and the primer design software Primer Primer5.0 was used to design primers. Avoid areas with high homology with other pathogens, manually adjust the parameters, the size of the amplification product is in the range of 150-300bp, and the Tm value of the primers is in the range of 54-64°C. A total of 20 pairs of primers were designed and selected, among which the 16S rRNA sequence 6 pairs of primers, 14 pairs of primers for ATPase sequence. The obtained prim...
Embodiment 2
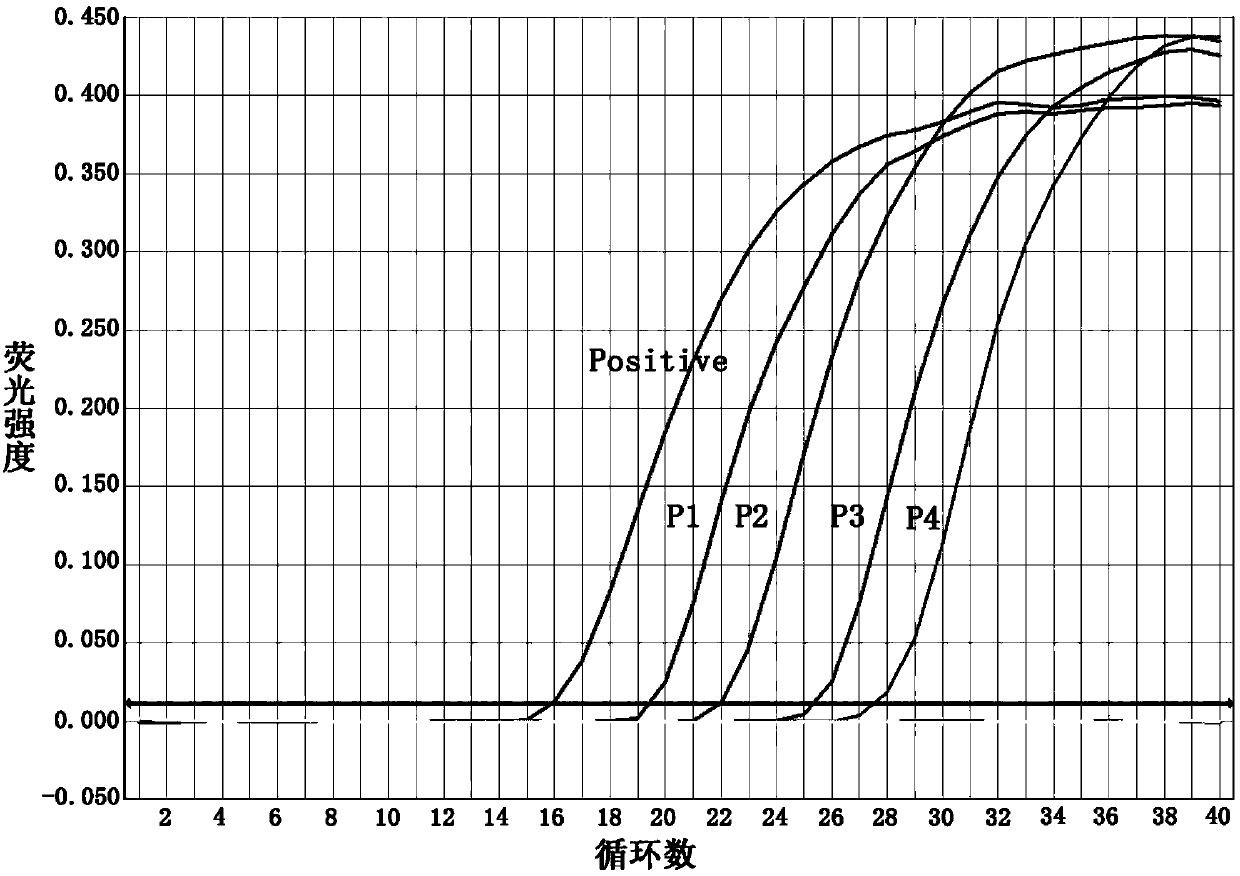
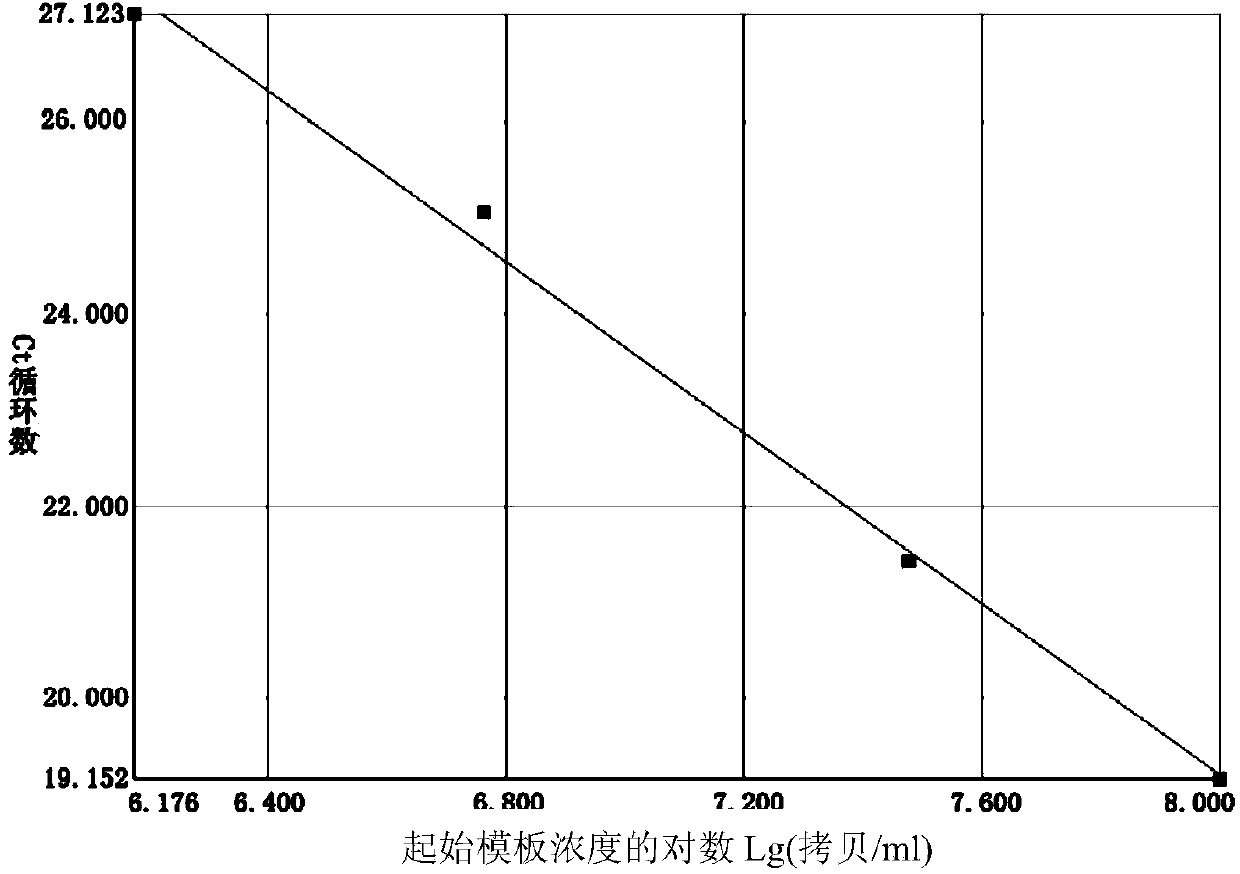
[0045] Embodiment 2, the specificity detection of the fluorescent PCR primer that is used to detect Mycoplasma pneumoniae and the determination of the minimum detection limit
[0046] In this example, the international standard strain of Mycoplasma pneumoniae FH (ATCC15531), four kinds of mycoplasma pathogens and 11 kinds of common respiratory tract bacteria and fungi were used to detect the specificity of the three primer pairs obtained in Example 1. Among them, four kinds of Mycoplasma pathogens are: Mycoplasma genitalium (ATCC33530), Mycoplasma hominis (ATCC23114), Ureaplasma urealyticum (ATCC27618) and Mycoplasma pirilum (ATCC25960); 11 common bacteria and fungi in the respiratory tract are: Haemophilus influenzae (hin) (ATCC49247), Streptococcus A (gas) (ATCC19615), Staphylococcus epidermidis (sep) (ATCC12228), Staphylococcus aureus (sau) (ATCC25923), Escherichia coli (eco) (ATCC25922) , Klebsiella pneumoniae (kpn) (ATCC700603), Moraxella catarrhalis (bca) (ATCC25238), Ac...
Embodiment 3
[0057] Embodiment 3, the clinical sample verification of the fluorescent PCR primer that is used to detect Mycoplasma pneumoniae
[0058] In this example, the inventor retrospectively analyzed the MP-related test results of 100 patients with community-acquired pneumonia (age ≥ 14 years old) in the fever clinic, and the MP-IgG antibody in the double serum was increased by 4 times or more as MP infection The "gold standard" of diagnosis, respectively evaluate the MP FQ-PCR product of Sun Yat-Sen University Daan Gene Co., Ltd. (catalogue number: DA-B064), the real-time fluorescent quantitative PCR method for MPP1 using the primers obtained in Example 1, and the detection of IgM antibody The detection rate, sensitivity, specificity, positive predictive value, negative predictive value and positive likelihood ratio of the method and culture method.
[0059] At the first outpatient visit (acute stage of infection) of each patient, throat swabs were collected and stored in 2ml of nor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com