Furopyridine compound as well as preparation method and application thereof
A furopyridine and compound technology, applied in the field of chemical medicine, can solve the problems of less colon cancer, lower cure rate and survival rate, and increased difficulty in cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
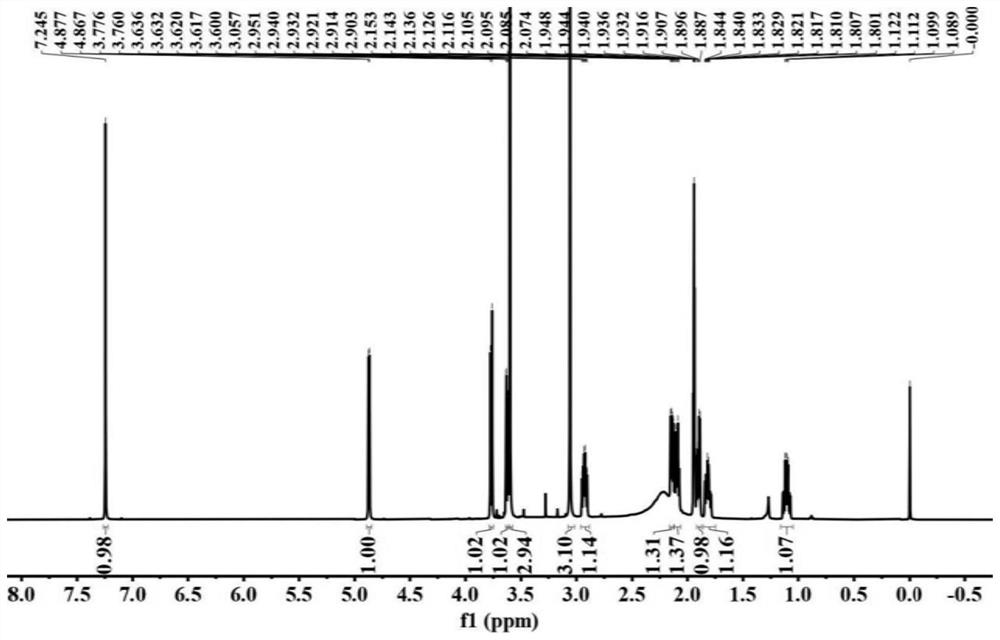
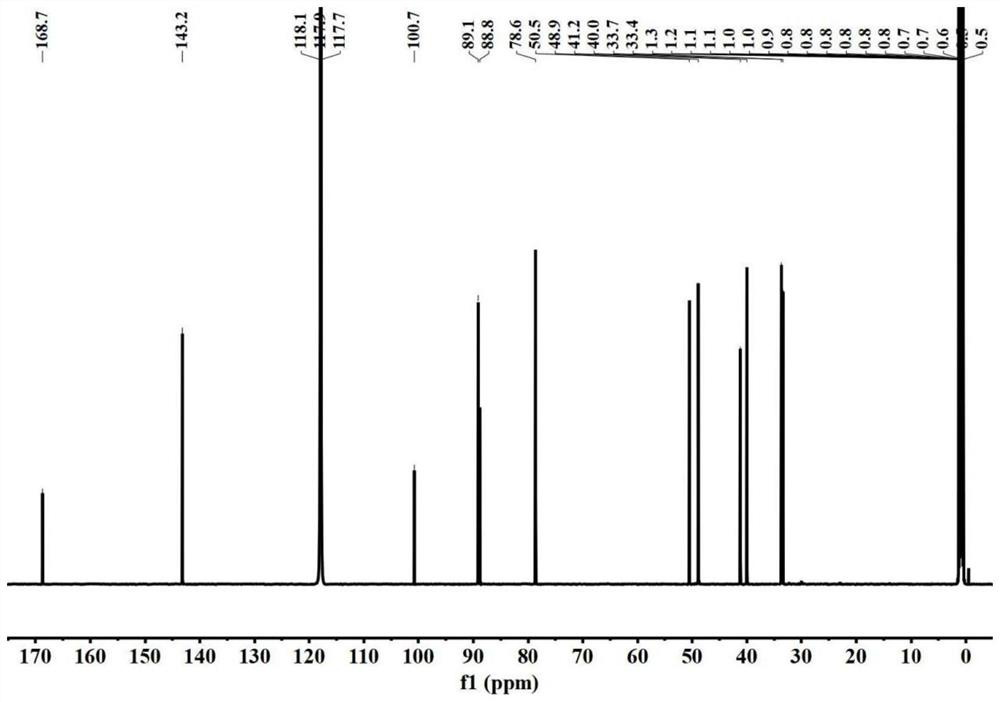
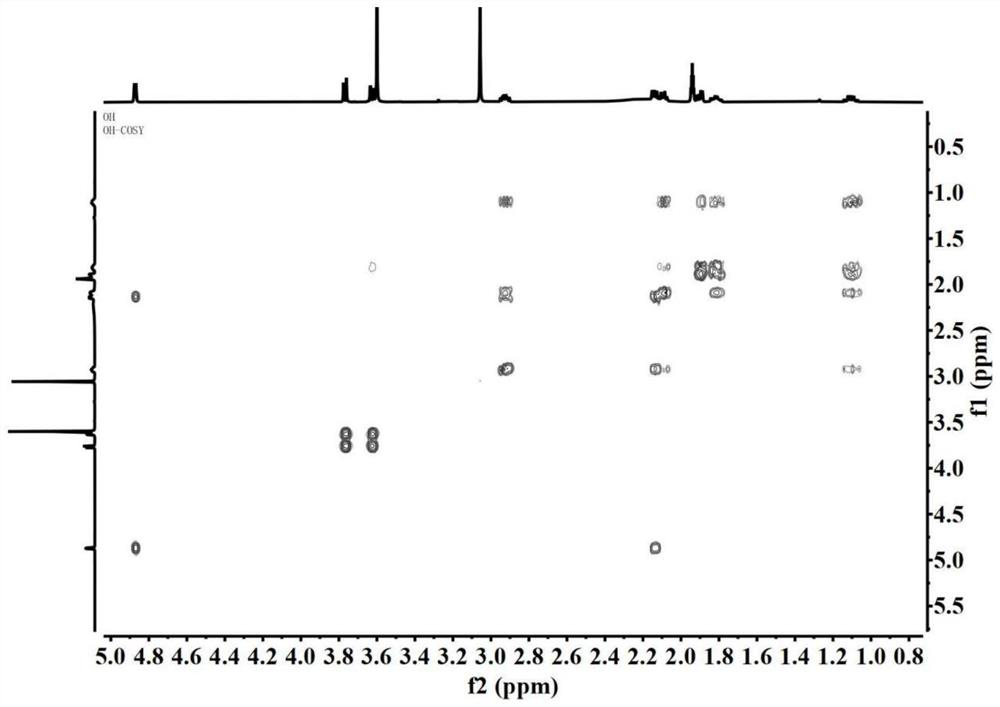
Image
Examples
preparation example Construction
[0034] The embodiment of the present invention also provides a preparation method of the compound, comprising the following steps:
[0035] Step A, dissolving genipin and methylamine in a non-nitrogen-containing buffer solution with a pH of 6-8, and reacting at 30-80° C. for more than 4 hours to obtain a reaction solution;
[0036] Step B, adding the reaction solution to the small-pore resin chromatography column, and successively using ethanol-water solvents with volume ratios of 0:10, 1:9, 2:8, 3:7, 4:6 to carry out gradient elution , and collect the eluate obtained by elution with an ethanol-water solvent with a volume ratio of 3:7 to 4:6, and then Genimethylamine A can be obtained in the eluate.
[0037] In this preparation method, the eluate obtained in step B can be further separated and purified by preparative liquid chromatography to obtain the pure product of Genimethylamine A. The purification method can be selected as follows: the eluate obtained in step B is separ...
Embodiment 1
[0046] This embodiment provides a furopyridine compound Genimethylamine A and a preparation method thereof.
[0047] The preparation method of Genimethylamine A specifically comprises the following steps:
[0048]Step A. Take 150 mg of genipin and 45 mg of methylamine respectively, accurately weigh them, place them in 150ml beakers, respectively add potassium dihydrogen phosphate-sodium hydroxide buffer solution of pH=6.8 to make them fully dissolved, and prepare them to the concentration of respectively. It is a stock solution of 4.4 mmol / L; pipette 150 ml of each of the above stock solutions, place them in a beaker, add a pH=6.8 potassium dihydrogen phosphate-sodium hydroxide buffer solution to 900 mL, shake well, and place in a 50 ℃ water bath, The reaction was taken out for 48h to obtain the reaction solution;
[0049] Step B, the reaction solution is separated by column chromatography using CHP20 / P120 small pore resin, and eluted with ethanol-water (0:10, 1:9, 2:8, 3:7, ...
Embodiment 2
[0056] This embodiment provides a preparation method of the furopyridine compound Genimethylamine A.
[0057] Specifically include the following steps:
[0058] Step A. Take 120 mg of genipin and 45 mg of methylamine respectively, accurately weigh them, and place them in a 150 mL beaker, respectively, and add potassium dihydrogen phosphate-sodium hydroxide buffer solution with pH=6.8 to fully dissolve them to prepare the concentration of 3.5 and 4.4 mmol / L stock solutions; pipette 150 mL of each of the above stock solutions, place them in a beaker, add pH=6.8 potassium dihydrogen phosphate-sodium hydroxide buffer solution to 900 mL, shake well, and place in a 45°C water bath , the reaction is taken out for 60h, and the reaction solution is obtained;
[0059] Step B, the reaction solution is separated by column chromatography using CHP20 / P120 small pore resin, and eluted with ethanol-water (0:10, 1:9, 2:8, 3:7, 4:6) solvent system gradient (3 column volumes for each elution s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com