Preparation of high-purity vancomycin hydrochloride
A technology for vancomycin hydrochloride and vancomycin, which is applied in the field of purifying vancomycin hydrochloride to achieve the effects of increasing effective components, reducing impurities and improving appearance and color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
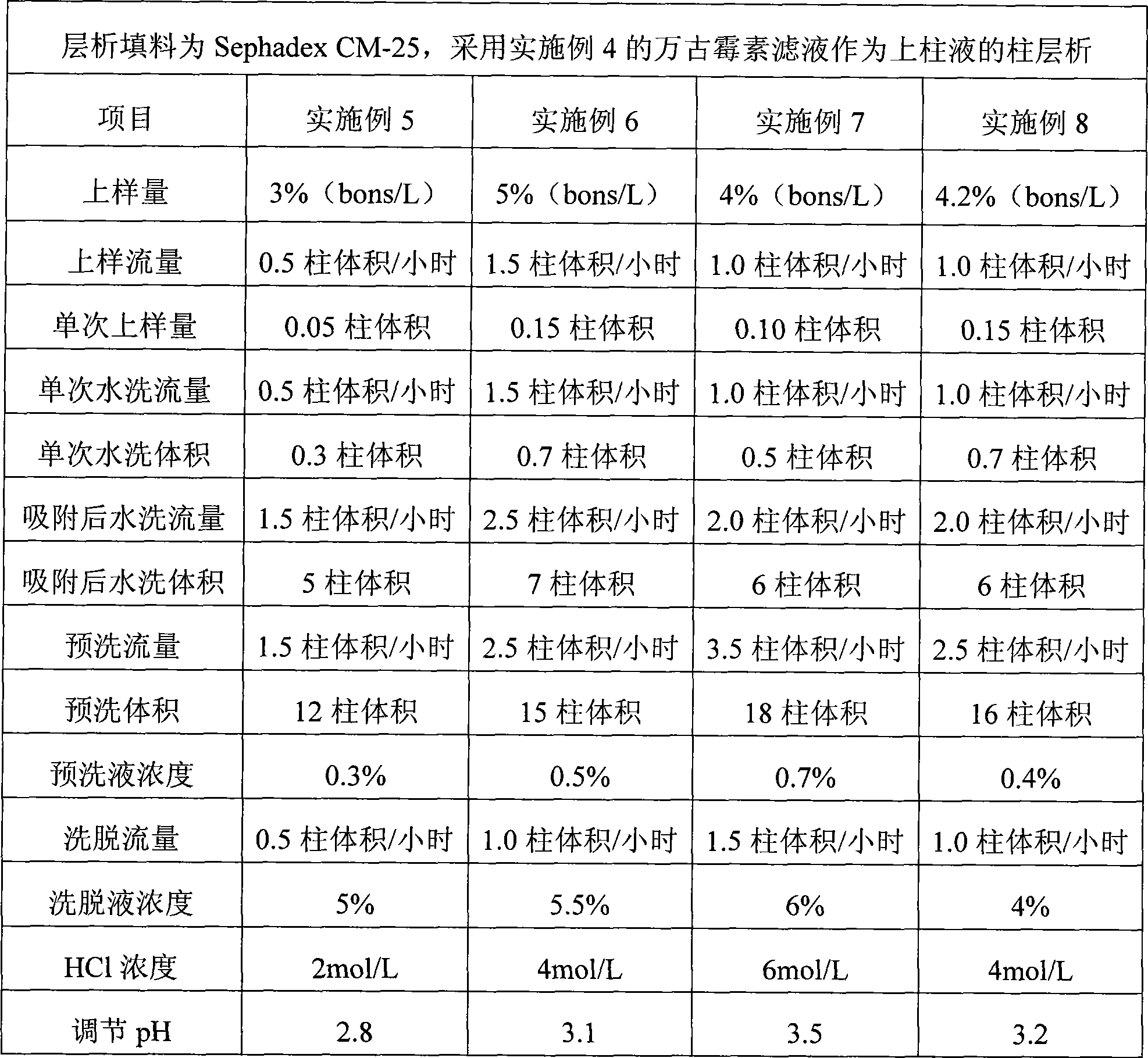
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of vancomycin crude product
[0032] The vancomycin hydrochloride aqueous solution has a concentration of 120 mg / mL, a volume of 20.0 L, and a vancomycin chromatographic purity of 80.4% (HPLC). The pH of the solution is 3.0, and NH is added while stirring. 4 HCO 3 A total of 1600g, during the process of adding salt, bubbles are constantly generated in the solution, and vancomycin is precipitated from it, forming a slurry, and then adding concentrated ammonia water to adjust the pH to 8.0, this means more vancomycin precipitates, and the slurry is thicker, continue Stir for 50min to make it evenly mixed, stop stirring and let stand for 18 hours, the process temperature is controlled at 14-16°C. The mixture was separated by filtration and top-washed with 5000 mL of ethanol to obtain 4023 g of a wet product of vancomycin base with a titer of 564 μg / mg and a yield of 94.5%. The chromatographic purity of vancomycin detected by vancomycin base ...
Embodiment 2
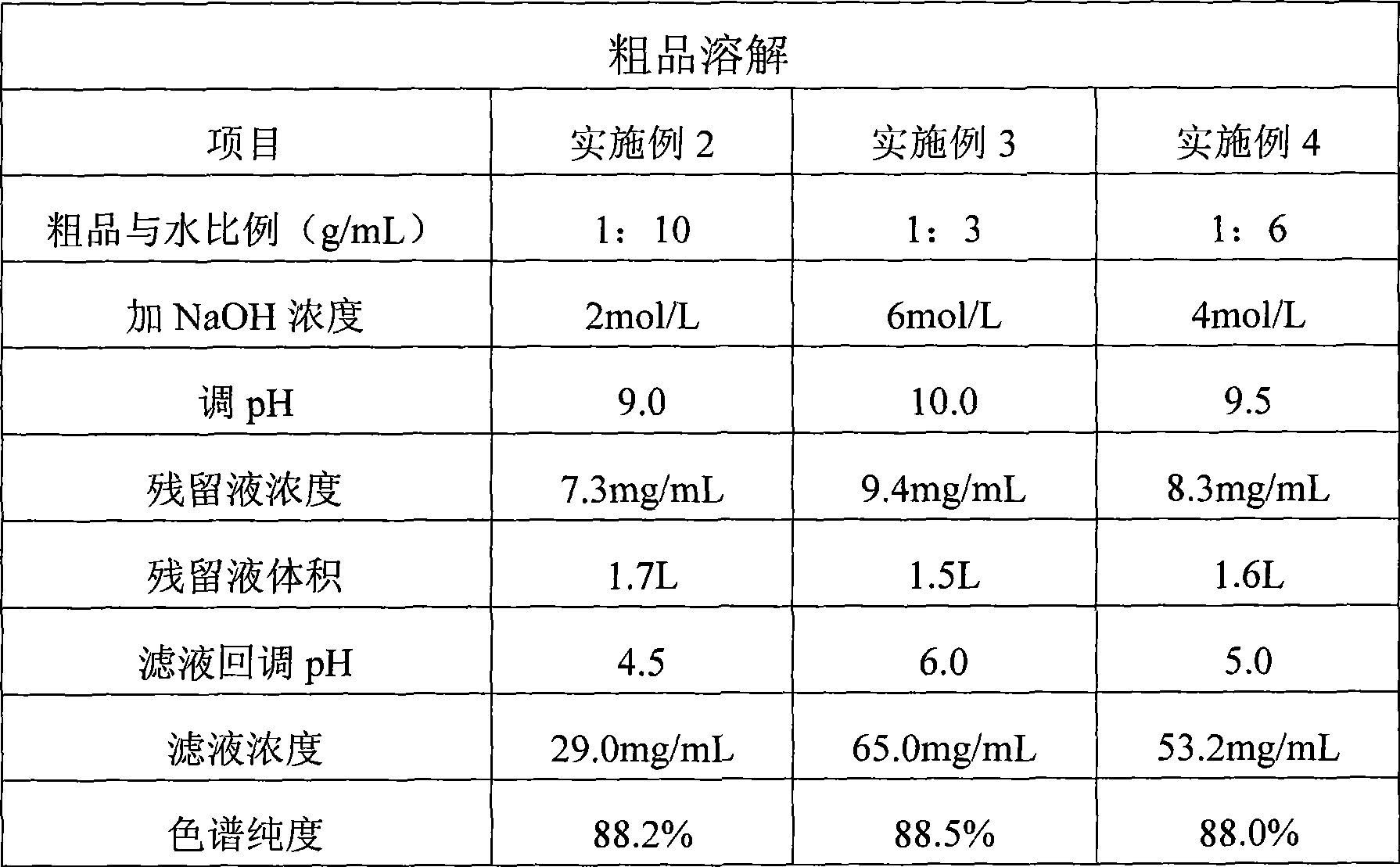
[0033] Embodiment 2: vancomycin crude product dissolves
[0034] In a glass beaker, 1000 g of crude vancomycin prepared in Example 1 was dissolved in 10.0 L of pure water, stirred, and adjusted to pH=9.0 with 2 mol / L NaOH solution. After complete dissolution, filter, add a small amount of pure water during the filtration, and stop the filtration when the concentration of vancomycin in the residue is 7.3mg / ml and the volume is 1.7L. Collect the filtrate in an acid-resistant reaction tank, and adjust the filtrate to pH=4.5 with 2mol / L HCl solution. Finally, 18.5 L of filtrate was obtained with a concentration of 29.0 mg / mL and a chromatographic purity of 88.2%. See Table 1.
Embodiment 3
[0035] Embodiment 3: vancomycin crude product dissolves
[0036] In a glass beaker, 1000 g of crude vancomycin prepared in Example 1 was dissolved in 3.0 L of pure water, stirred, and adjusted to pH=10.0 with 6 mol / L NaOH solution. After complete dissolution, filter, add a small amount of pure water during the filtration, stop the filtration when the concentration of vancomycin in the residue is 9.4mg / ml and the volume is 1.5L. Collect the filtrate in an acid-resistant reaction tank, and adjust the filtrate to pH=6.0 with 6mol / L HCl solution. Finally, 8.5 L of filtrate was obtained with a concentration of 65.0 mg / mL and a chromatographic purity of 88.5%. See Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Chromatographic purity | aaaaa | aaaaa |
| Chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com