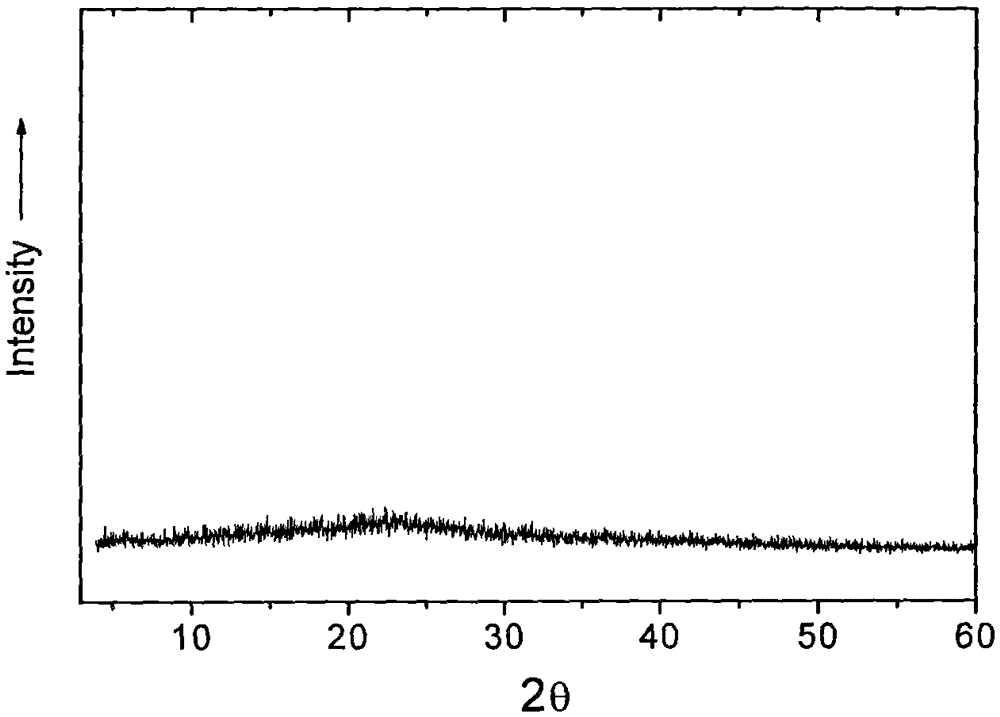
Non-crystalline vancomycin hydrochloride, its preparation method and use, and its pharmaceutical composition
A technology of vancomycin hydrochloride and vancomycin, which is applied in the direction of drug combination, anti-infective drugs, glycopeptide components, etc., can solve the problems of large capsule particles and difficulty in taking, and achieve the improvement of effective components, appearance and color change, and impurities Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of crude vancomycin
[0035] The vancomycin fermentation broth was filtered under alkaline conditions, and the obtained clarified filtrate was passed through the macroporous adsorption resin XAD-1600. Vancomycin was adsorbed on the resin. After the resin was washed with water, it was eluted with an acidic aqueous solution of 40% ethanol. After elution, the eluate containing vancomycin was added to activated carbon for decolorization, the filtrate obtained by filtration was concentrated to obtain an aqueous solution of vancomycin hydrochloride, and then NH was added. 4 HCO 3 Precipitation was carried out to obtain crude vancomycin. The vancomycin B content in the crude vancomycin product was 86.8%, and the titer was 599u / mg.
[0036] Take 2500g of this crude product, add 20.0L of purified water, add NaOH solution to adjust the pH to 10.0, use 0.2M 2 The 0.5μm pore size ceramic membrane was filtered to obtain 25.0L vancomycin filtrate, the soluti...
Embodiment 2
[0037] Example 2: Precipitation of Vancomycin Hydrochloride
[0038] Take 500 mL of the concentrated solution in Example 1, filter it through 0.45 μm and 0.2 μm filters, and add 25 g of NaHCO while stirring. 3 , Vancomycin was precipitated from it to form a slurry, add HCl aqueous solution to adjust the pH to 2.80, continue to stir for 60min to make it evenly mixed, stop stirring and let stand for 38 hours, the process temperature is controlled at 14 ~ 15 ℃. The mixture was separated by filtration and firstly washed with 200 mL of 90% ethanol. After the top washing, the conductivity of the top washing liquid was 385us / cm, and then top washed with 200 mL of absolute ethanol to obtain 99.7 g of vancomycin hydrochloride wet product. After removing the residual ethanol by drying, 72.5 g of the intermediate was obtained, and then 63.2 g of finished vancomycin hydrochloride was obtained after drying in high vacuum. The moisture content of the finished product was 3.4%. g / ml. The n...
Embodiment 3
[0039] Example 3: Precipitation of Vancomycin Hydrochloride
[0040] Take 500 mL of the concentrated solution in Example 1, filter it through 0.45 μm and 0.2 μm filters, and add 15 g of NaHCO while stirring. 3 , Vancomycin was precipitated from it to form a slurry, add HCl aqueous solution to adjust the pH to 3.50, continue to stir for 30min to make it evenly mixed, stop stirring and let stand for 48 hours, the process temperature is controlled at 5 ~ 6 ℃. The mixture was separated by filtration and firstly washed with 200 mL of 90% ethanol. After the top washing, the conductivity of the top washing liquid was 293 us / cm, and then top washed with 200 mL of absolute ethanol to obtain 92.3 g of vancomycin hydrochloride wet product, wet product. The product is dried to remove residual ethanol to obtain 68.5g of intermediate, and then dried in high vacuum to obtain 58.6g of finished vancomycin hydrochloride, the moisture content of the finished product is 3.1%, the bulk density of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com