Method for preparing high-purity samples of impurities of vancomycin hydrochloride
A vancomycin hydrochloride, high-purity technology is applied in the field of high-purity sample preparation, which can solve the problems of low content, high preparation cost, and consumption of a large amount of vancomycin hydrochloride, and achieve the effect of simple process and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Take 6g of vancomycin hydrochloride crystalline powder, add purified water to prepare a 10g / L aqueous solution, and the volume of the solution is about 600mL;
[0038] 2. Heat the solution prepared in step 1 in a water bath at a temperature of 30°C; add 42mL of ethylene glycol, stir evenly and keep warm for 122 hours;
[0039] 3. Cool down to 20°C, slowly add 3852mL of absolute ethanol dropwise to crystallize, and centrifuge to separate the crystals;
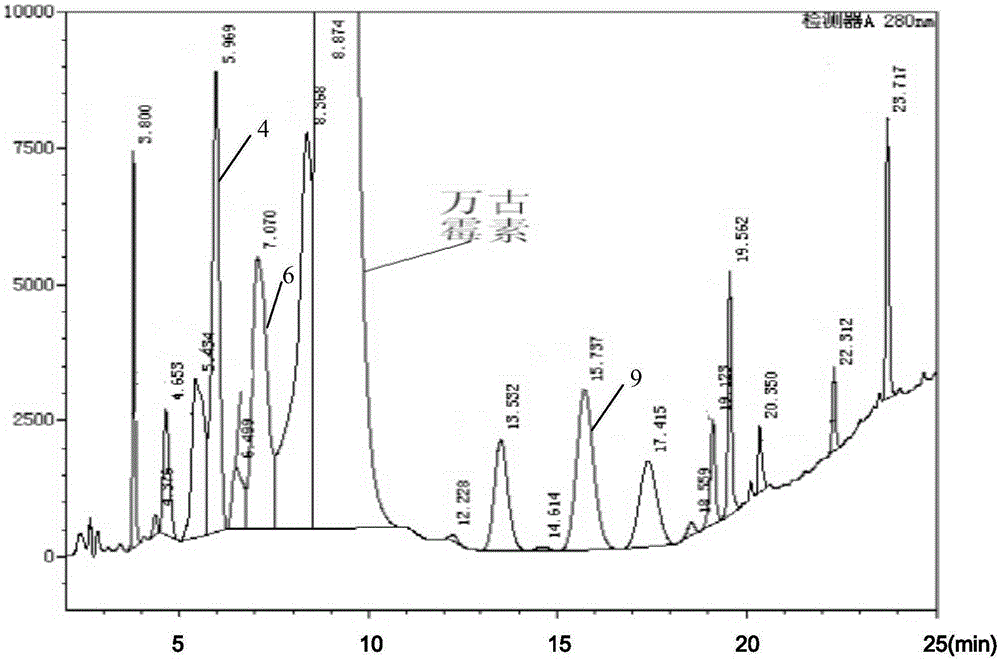
[0040] 4. High-pressure liquid chromatography detects crystals, the content of impurity 4 is 17.68% (mass percentage, the same below), the content of impurity 6 is 21.4%, and the content of impurity 9 is 6.97%;
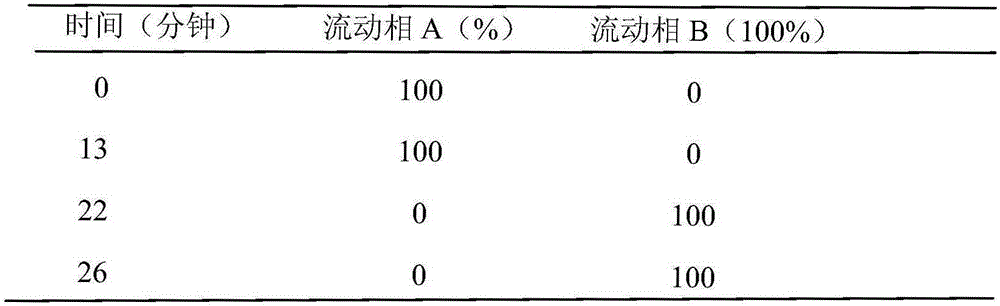
[0041] 5. Use high-pressure preparative liquid chromatography for preparative separation, separate impurities for freeze-drying detection, and the results are shown in Table 3.
[0042] table 3
[0043] Impurity name Chromatographic purity freeze-dried weight Impurity 4 98.5% 246μg ...
Embodiment 2
[0045] 1. Take 8g of vancomycin hydrochloride crystalline powder, add purified water to make a 1.5g / L aqueous solution, and the volume of the solution is about 533mL;
[0046] 2. Heat the solution prepared in step 1 in a water bath at a temperature of 33°C; add 42.6mL of ethylene glycol, stir evenly and keep warm for 120 hours;
[0047] 3. Cool down to 21°C, slowly add 2302mL of absolute ethanol dropwise to crystallize, and centrifuge to separate the crystals;
[0048] 4. The crystals were detected by high-pressure liquid chromatography, and the content of impurity 4 was 15.32%, the content of impurity 6 was 19.36%, and the content of impurity 9 was 10.67%;
[0049] 5. Use high-pressure preparative liquid chromatography for preparative separation, and separate impurities for freeze-drying detection. The results are shown in Table 4.
[0050] Table 4
[0051] Impurity name Chromatographic purity freeze-dried weight Impurity 4 98.6% 186μg Impurity 6 ...
Embodiment 3
[0053] 1. Take 5g of vancomycin hydrochloride crystalline powder, add purified water to make a 1g / L aqueous solution, and the volume of the solution is about 500mL;
[0054] 2. Heat the solution prepared in step 1 in a water bath at a temperature of 33°C; add 35mL of ethylene glycol, stir evenly and keep warm for 120 hours;
[0055] 3. Cool down to 21°C, slowly add 2675mL of absolute ethanol dropwise to crystallize, and centrifuge to separate the crystals;
[0056] 4. The crystals were detected by high-pressure liquid chromatography. The content of impurity 4 was 16.73%, the content of impurity 6 was 22.34%, and the content of impurity 9 was 11.65%;
[0057] 5. Use high-pressure preparative liquid chromatography for preparative separation, separate impurities for freeze-drying detection, and the results are shown in Table 5.
[0058] table 5
[0059] Impurity name Chromatographic purity freeze-dried weight Impurity 4 97.8% 173μg Impurity 6 98.3% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com