Drug-carried nanoparticle, aerogel and preparation method and application thereof
A drug-loaded nanometer and hydrogel technology, which is applied in the fields of pharmaceutical formulation, medical science, capsule delivery, etc., can solve the problems of limited application, achieve the effect of improving the lesion site, increasing the clinical cure rate, and reducing postoperative complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
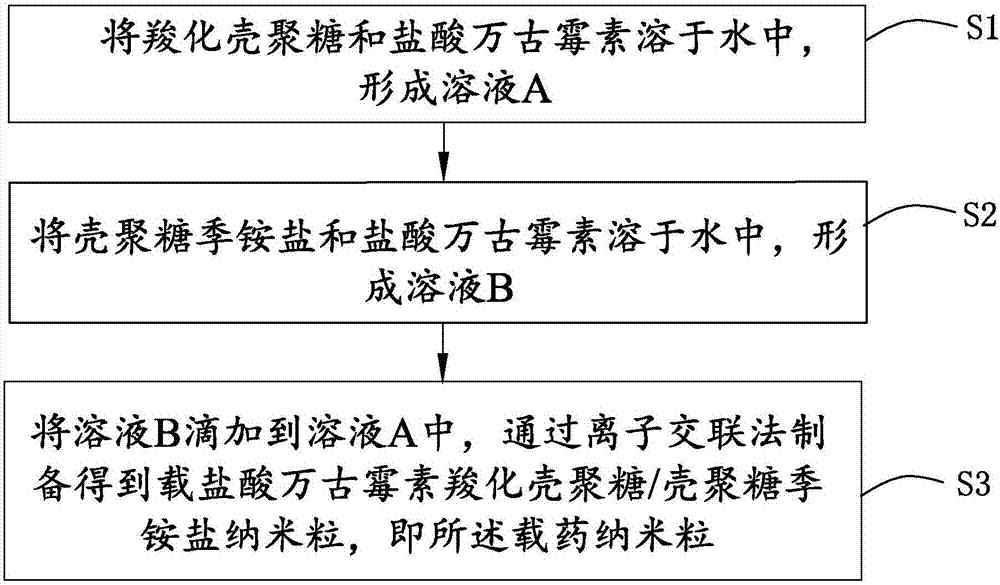
[0066] see figure 1 , is a schematic flow chart of the preparation method of the drug-loaded nanoparticles provided by the present invention. The preparation method of the drug-loaded nanoparticles comprises the following steps:
[0067] Step S1: Dissolve an appropriate amount of CC and 5 mg of VCM in 10 mL of water to form a solution A;
[0068] Step S2: Dissolving an appropriate amount of QAC and 1.8 mg of VCM in 8 mL of water to form solution B;
[0069] Step S3: Add solution B dropwise to solution A, and prepare vancomycin hydrochloride-loaded carboxylated chitosan / chitosan quaternary ammonium salt nanoparticles (hereinafter referred to as VCM / CC-QAC-NPs) by ion cross-linking , namely the drug-loaded nanoparticles.
[0070] Be 6.8 mg by fixing the amount of VCM, the mass ratio of carboxylated chitosan and chitosan quaternary ammonium salt is respectively set to 10:1, 10:2, 10:3, 10:4, 10:5, obtains VCM / CC-QAC-NPs of different schemes form Examples 1-5.
[0071] The fo...
Embodiment 6
[0097] see Figure 5 , is a schematic flow chart of the preparation method of the hydrogel provided by the present invention. The preparation method of described hydrogel comprises the steps:
[0098] Step S1: Prepare a GP aqueous solution, and add 1 mL of the drug-loaded nanoparticles described in Example 4 into the aqueous sodium glycerophosphate solution, and mix evenly to obtain a solution C;
[0099] Specifically, GP is dissolved in 1 mL of ionized water, wherein GP can be α-GP or / and β-GP, preferably a mixture of α-GP and β-GP; in this embodiment, α-GP / β-GP ( w / w)=1:2-8,
[0100] Step S2: Dissolve an appropriate amount of CS and QAC in 0.1M acetic acid, and stir to obtain a clear solution D;
[0101] Specifically, CS / GP(w / w)=1.2-5:25; the solvent may be other organic carboxylic acids, sulfonic acids, sulfinic acids, etc. in addition to acetic acid.
[0102] Step S3: Add solution C dropwise to solution D in an ice bath, and stir for 15 minutes to obtain a drug-loaded ...
Embodiment 7-10
[0107] Using the hydrogel preparation method of Example 6, taking the amount of drug-loaded nanoparticles, CS / GP (w / w)=2:25 as quantitative, by changing the mass ratio of α-GP / β-GP, the example 7-10 VCM / CC-QAC-NPs / CS-QAC-Gel, and investigate its gel time at 37°C, as shown in Table 3. and please refer to Figure 7 , is a histogram of the gel time detection results of Examples 7-10 in the preparation method of the hydrogel provided by the present invention.
[0108] Table 3: Effect of α-GP / β-GP (w / w) on Gel Time
[0109] α-GP / β-GP (w / w) Gel time (min) Example 7 1:2 3.40 Example 8 1:4 3.6 Example 9 1:6 3.65 Example 10 1:8 4.3
[0110] combine Figure 7 It can be seen from Table 3 that the gelation time gradually increases with the decrease of the amount of α-GP, indicating that α-GP is beneficial to shorten the gelation time of the gel, and α-GP / β-GP=1:2(w / w) The minimum gelling time is 3.40min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com