Carbamic acid ester type liquid phase chromatogram stationary phase and preparation method thereof
A high-performance liquid chromatography and stationary phase technology, applied in the field of novel reversed-phase liquid chromatography stationary phase, can solve the problems of cumbersome preparation process of stationary phase, expensive reagents, etc., achieve good market application prospects, improve compatibility, and preparation process Simple and reliable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
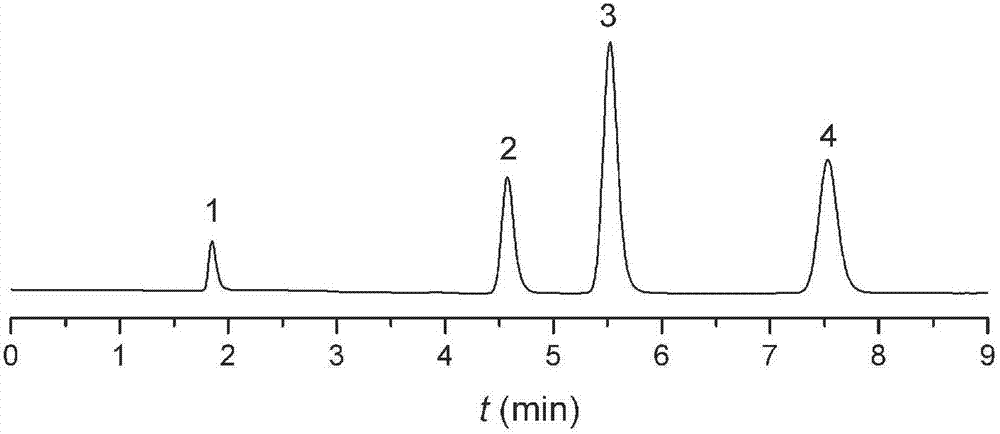
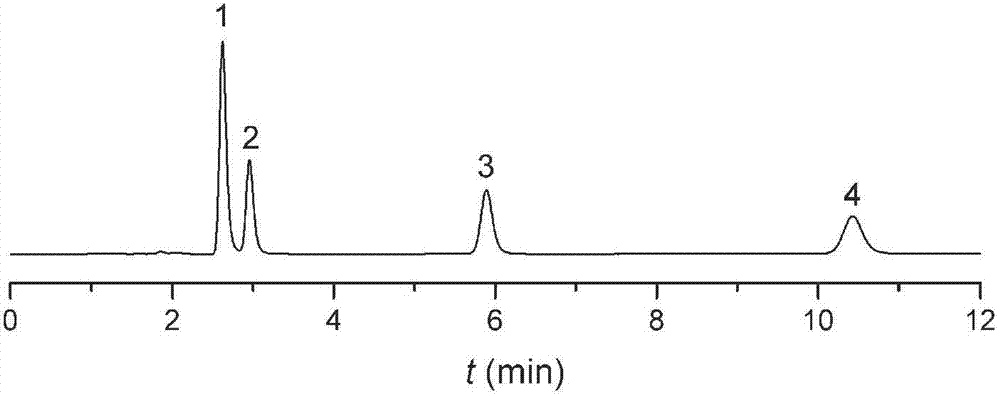
Image
Examples
Embodiment 1
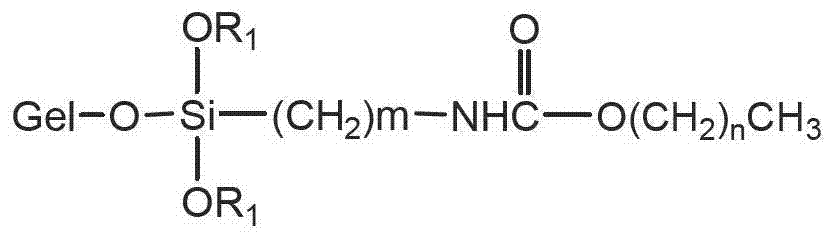
[0022] Add 5 mL of propyltriethoxysilane isocyanate, 3 g of dodecanol and 40 mL of toluene into a 100 mL flask, heat to reflux for 3 hours, cool and dry for later use. Take another 2.5g of silica gel treated with hydrochloric acid in a 200mL flask, dry it in vacuum at 150°C for 5 hours, add the above reaction solution under vacuum drive, stir and reflux for 14 hours under the protection of nitrogen, filter, and wash with toluene and methanol successively. The obtained solid was dried in a vacuum oven at 80° C. for 12 hours to obtain a carbamate chromatographic stationary phase. Elemental analysis: C: 13.2%, N: 0.9%. Infrared spectrum: 2930 and 2858cm -1 Alkyl characteristic absorption peak, 1703cm -1 Carbonyl characteristic absorption peak, 1530cm -1 The characteristic absorption peak of the amide bond. The results of elemental analysis and infrared spectroscopy confirmed that the structure of the stationary phase is:
[0023]
Embodiment 2
[0025] Add 30mL of propyltriethoxysilane isocyanate, 25g of dodecanol and 250mL of toluene into a 500mL flask, heat to reflux for 3 hours, cool and dry for later use. Take another 30g of silica gel treated with hydrochloric acid in a 1000mL flask, dry it in vacuum at 150°C for 5 hours, add the above reaction solution under vacuum drive, stir and reflux for 14 hours under the protection of nitrogen, filter, and wash with toluene and methanol in turn to obtain The solid was dried in a vacuum oven at 80° C. for 12 hours to obtain a carbamate chromatographic stationary phase. Elemental analysis: C: 11.8%, N: 0.8%. Infrared spectrum: 2930 and 2859cm -1 Alkyl characteristic absorption peak, 1703cm -1 Carbonyl characteristic absorption peak, 1533cm -1 The characteristic absorption peak of the amide bond. The results of elemental analysis and infrared spectroscopy confirmed that the structure of the stationary phase is:
[0026]
Embodiment 3
[0028] The difference from Example 1 is that stearyl alcohol is used instead of dodecanol. Elemental analysis: C: 16.3%, N: 0.8%. Infrared spectrum: 2930 and 2858cm -1 Alkyl characteristic absorption peak, 1703cm -1 Carbonyl characteristic absorption peak, 1530cm -1 The characteristic absorption peak of the amide bond. The results of elemental analysis and infrared spectroscopy confirmed that the structure of the stationary phase is:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com