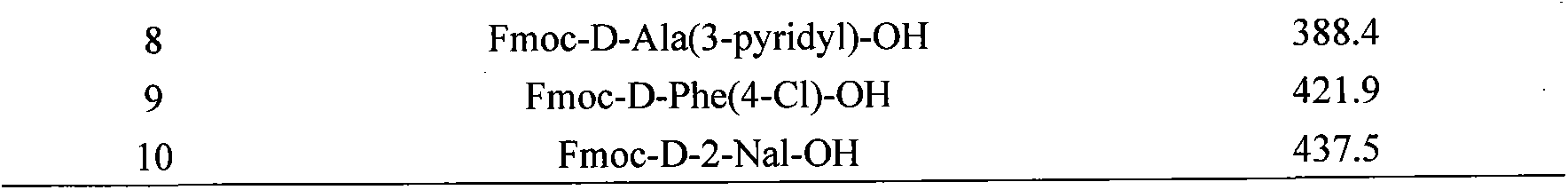Method for preparing cetrorelix acetate by taking Rink Amide-AM Resin as carrier
A technology of cetrorelix acetate and cetrorelix, which is applied in the field of polypeptide drug preparation and can solve the problems of great harm to human body and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Swelling of amino resin
[0026] Weigh 0.16g of Rink-AM-Resin with a degree of substitution of 0.3-0.87mmol / g, add it to the polypeptide synthesis reactor from the open end, take the DCM reagent and add it to the reactor, so that the resin is completely immersed in the DCM solvent. Fully exposed to the solvent, swelling for 2h.
[0027] 2. Synthesis of Fmoc-cetrorelix resin
[0028] The cetrorelix precursor peptide I-amino resin is:
[0029] Fmoc-D-Nal-D-Cpa-D-Pal-Ser-Tyr-D-Cit-Leu-Arg-Pro-D-Ala-Amino Resin
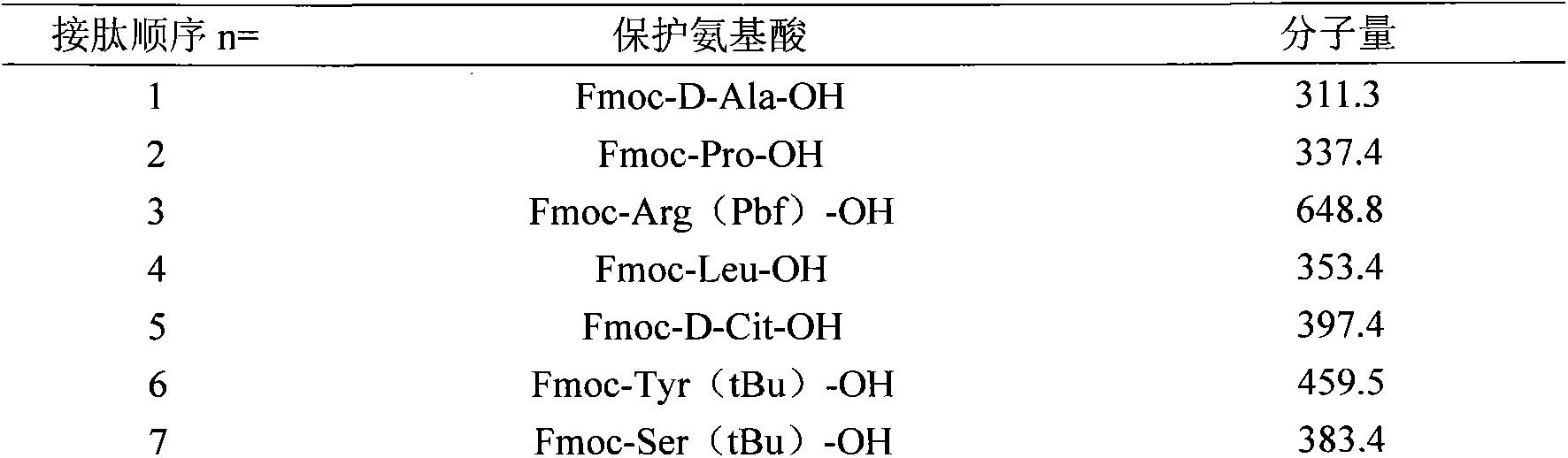
[0030] The protected amino acids used in this example are listed in the table below for the protected amino acids and molecular weights corresponding to the 1st to 10th amino acids from the resin:
[0031]
[0032]
[0033] Some commonly used abbreviations in the present invention have the following meanings:
[0034] Fmoc: fluorenylmethoxycarbonyl pbf: 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl
[0035] D-Cit: D-citrulline tBu: tert-butyl 3-py...
Embodiment 2
[0052] The present invention has carried out the comparative experiment of the yield of the preparation method of cetrorelix, namely condensation reaction 1) washing after normal condensation reaction 20-50min; 25-30min after normal condensation reaction, adding 0.5ml DIEA, and washing after microwave amino resin The yield of the cetrorelix crude product synthesized through the above-mentioned experimental method is ① 92.7%, ② 95.2%, and the cetrorelix acetate pure product ① 23.3mg, ② 25.8mg are obtained through purification and salt conversion. Product yields were ①30.8%, ②34.1%.
Embodiment 3
[0054] In order to determine the stability of the experimental results, the present invention has carried out two times of repeated synthesis, condensation reaction ① normal condensation reaction 20 ~ 50min after washing ② normal condensation reaction 25 ~ 30min, add 0.5ml DIEA, microwave amino resin after 10 ~ 20s Washing; the purity of the cetrorelix crude product is 78-85%, the yield of the crude product is 1.93.7%, 2.94.98%, and the pure cetrorelix acetate 1.31.7mg, 2.34. 76mg, the pure product yields are ①31.01%, ②34.0%; confirm the correctness and stability of the experimental data of the present invention.
[0055] According to the above experiments in Example 2 and Example 3, it can be concluded that the pure product of cetrorelix acetate prepared by microwave after normal synthesis has higher yield and better effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com