Salt conversion method of cetrorelix
A technology of cetrorelix and salt transfer, applied in the field of biological drug preparation, can solve problems such as poor salt transfer effect and trifluoroacetic acid residue, and achieve the effects of low temperature and high replacement efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
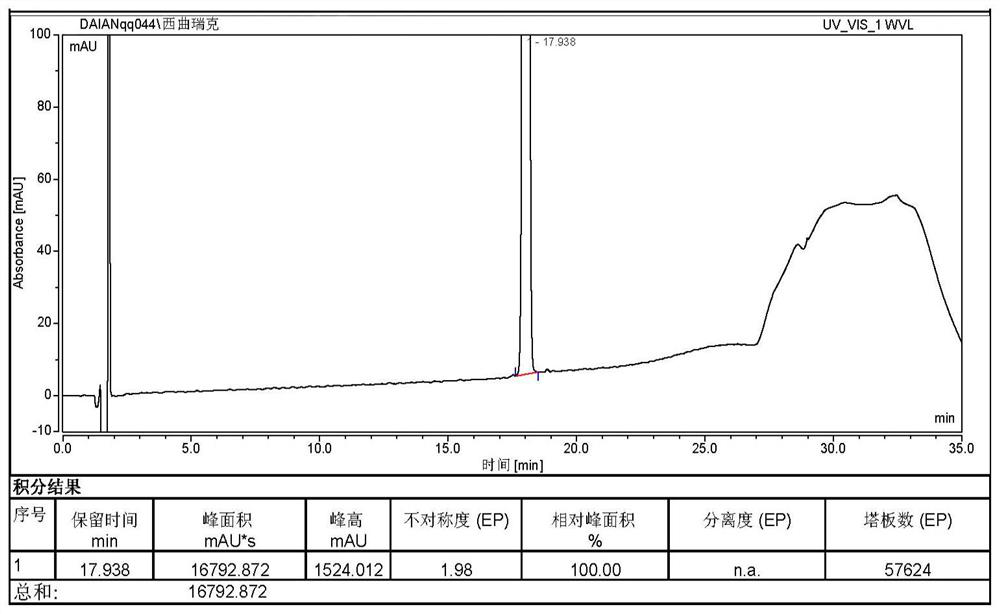
Image
Examples
Embodiment 1
[0031] The salt conversion method of Cetrorelix of the present invention, the detailed steps of described salt conversion method are as follows:
[0032] a. Transfer 32.0g of cetrorelix crude peptide into a beaker, put into a stirring bar, add 80ml of acetonitrile solution containing 0.1% trifluoroacetic acid, stir to dissolve it, then add 720ml of 0.1% trifluoroacetic acid aqueous solution, stir Until the solution is clear, the crude peptide solution is obtained; the obtained crude peptide solution is filtered through a 0.45 μm water filter membrane, and the filtrate is collected;
[0033] b. The filtrate obtained in step a is purified by reverse-phase chromatography using a chromatographic column, and the target peak eluent is collected to obtain a cetrorelix solution;
[0034] Adopt chromatographic column to carry out the condition in reversed-phase chromatographic purification process as follows:
[0035] Rinse the preparative column with filtered purified water for 5 min...
Embodiment 2
[0047] The salt conversion method of Cetrorelix of the present invention, the detailed steps of described salt conversion method are as follows:
[0048] a. Transfer 16.0 g of cetrorelix crude peptide into a beaker, put it into a stirring bar, add 80 ml of acetonitrile solution containing 0.1% trifluoroacetic acid, stir to dissolve it, then add 720 ml of 0.1% trifluoroacetic acid aqueous solution, and stir Until the solution is clear, the crude peptide solution is obtained; the obtained crude peptide solution is filtered through a 0.45 μm water filter membrane, and the filtrate is collected;
[0049] b. The filtrate obtained in step a is purified by reverse-phase chromatography using a chromatographic column, and the target peak eluent is collected to obtain a cetrorelix solution;
[0050] Adopt chromatographic column to carry out the condition in reversed-phase chromatographic purification process as follows:
[0051] Rinse the preparative column with filtered purified water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com