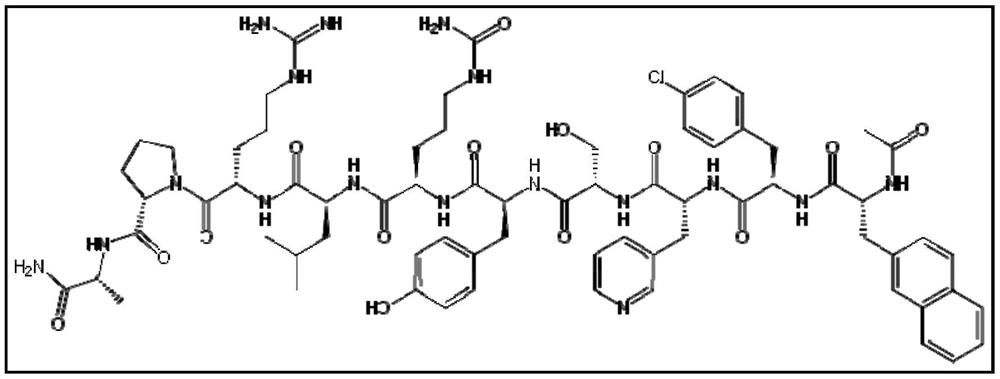
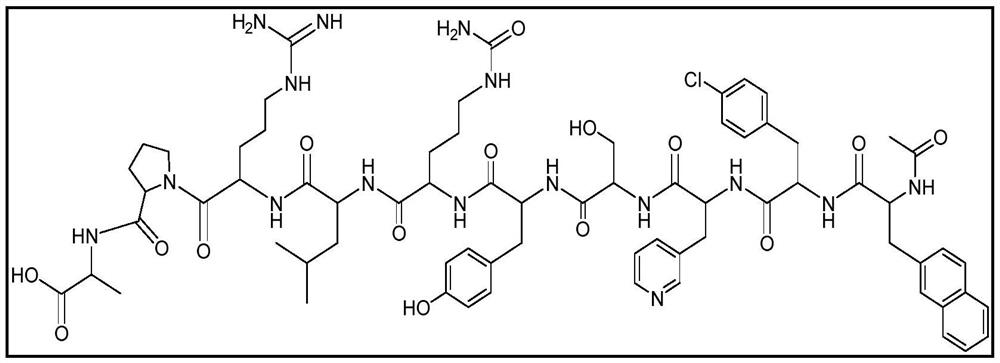
Stable formulation of cetrorelix
A technology of cetrelix and preparations, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0067] The invention has been described by way of example only, and modifications thereto will be recognized as falling within the scope and spirit of the appended claims and as would be apparent to those skilled in the art based on the disclosure herein, and are considered included within the scope of the present invention.
example -1
[0068] Example-1 : Stable formulation of cetrorelix.
[0069] Numbering Element Volume (%w / v) 1 cetrorelix acetate equivalent to cetrorelix 0.01%-10% 2 Cyclodextrin 0.1%-30% 3 tonicity agent 0%-10% 4 Surfactant 0.001%-1.0% 5 glacial acetic acid* qs to pH adjustment 6 Water for Injection Moderate to 100%
[0070] * Not more than 0.05 μg / ml (ie 0.025 μg / 0.5ml).
[0071] Manufacturing method:
[0072] a) Water for injection is transferred to a stainless steel vessel, and nitrogen is sprayed into the water,
[0073] b) adding cyclodextrin to the solution and stirring the solution,
[0074] c) Add the tonicity agent, surfactant and cetrorelix acetate to the solution obtained above with stirring until it dissolves,
[0075] d) adjusting the desired pH of the solution with glacial acetic acid,
[0076] e) Filter the solution and fill the sterile solution into a pre-filled syringe.
example -2
[0077] Example-2 : Stable formulation of cetrorelix (0.5 mg / ml).
[0078] Numbering Element Quantity mg / ml Volume (%w / v) 1 cetrorelix acetate equivalent to cetrorelix 0.5mg 0.05% 2 Hydroxypropyl beta cyclodextrin 200mg 20% 3 Mannitol 20mg 2% 4 Polysorbate 80 0.01mg 0.001% 5 glacial acetic acid* qs to pH adjustment qs to pH adjustment 6 Water for Injection Appropriate amount to 1.0ml Moderate to 100%
[0079] * Not more than 0.05 μg / ml (ie 0.025 μg / 0.5ml).
[0080] Example-2 can be prepared by the method described in Example-1 above.
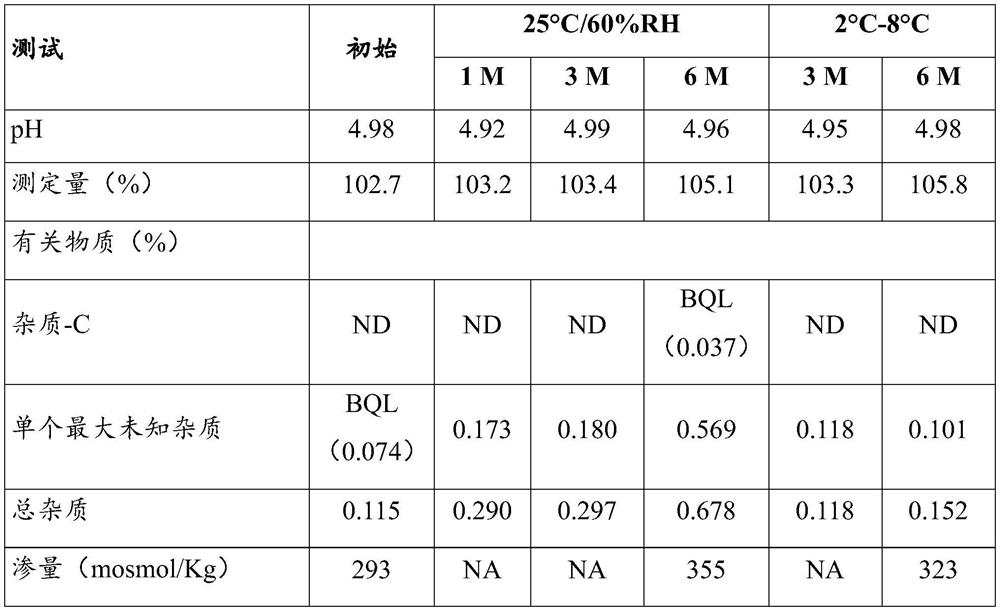
[0081] The results of the unfiltered and filtered bulk are shown in the table below:
[0082] Numbering test unfiltered ontology filtered ontology 1 describe clear colorless solution clear colorless solution 2 Measured amount 102.3% 102.7%
[0083] Visual observation and measurement results showed that the cetrorelix acetate formu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com