Biomechanical experiment simulation device for implantation of intravascular stent
A biomechanical and experimental simulation technology, used in stents, teaching models, medical science, etc., can solve problems such as stent collapse, poor adherence, and poor material biocompatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be further described below in conjunction with the accompanying drawings, but the protection scope of the present invention is not limited to the scope described in the embodiments.
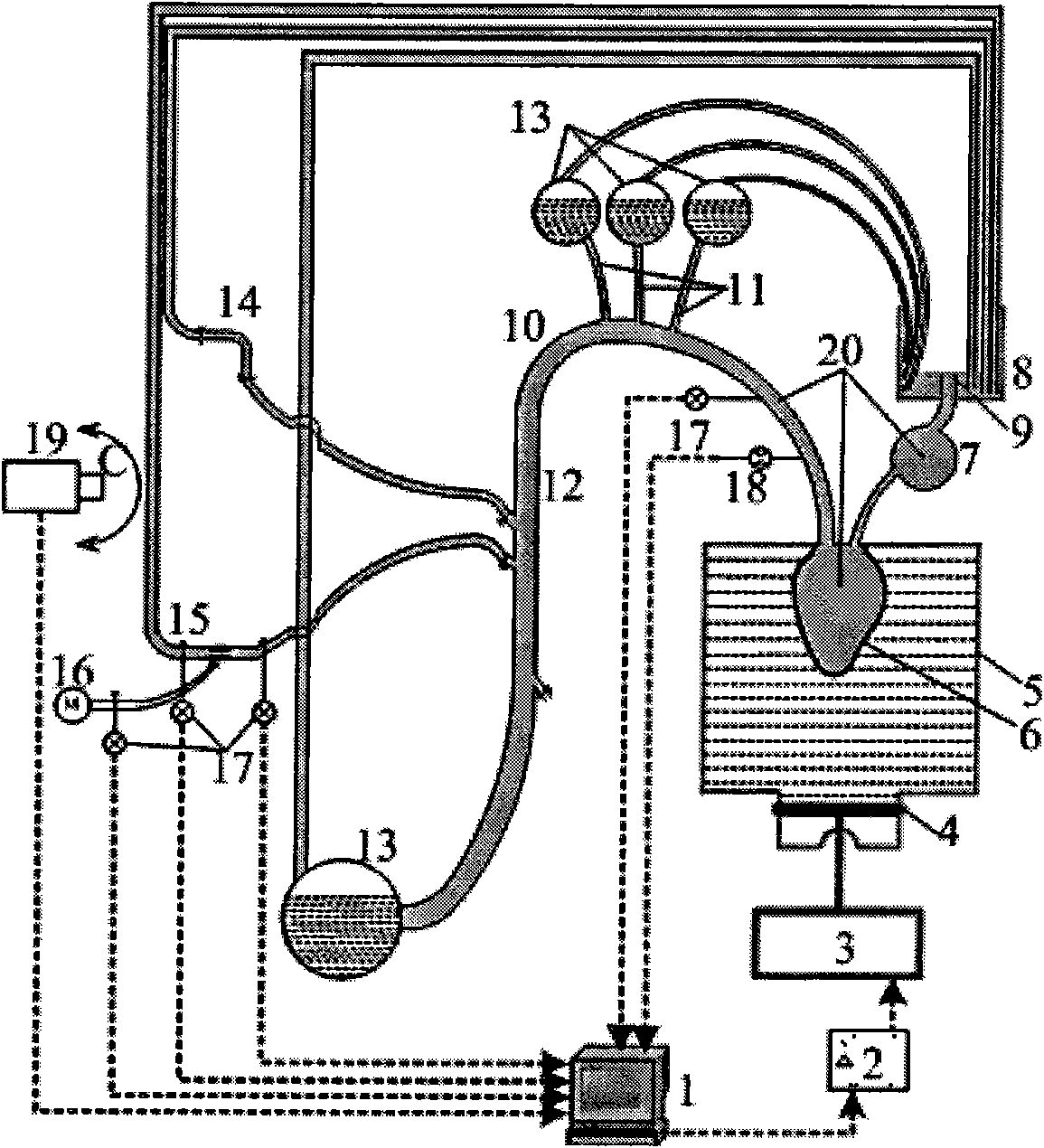
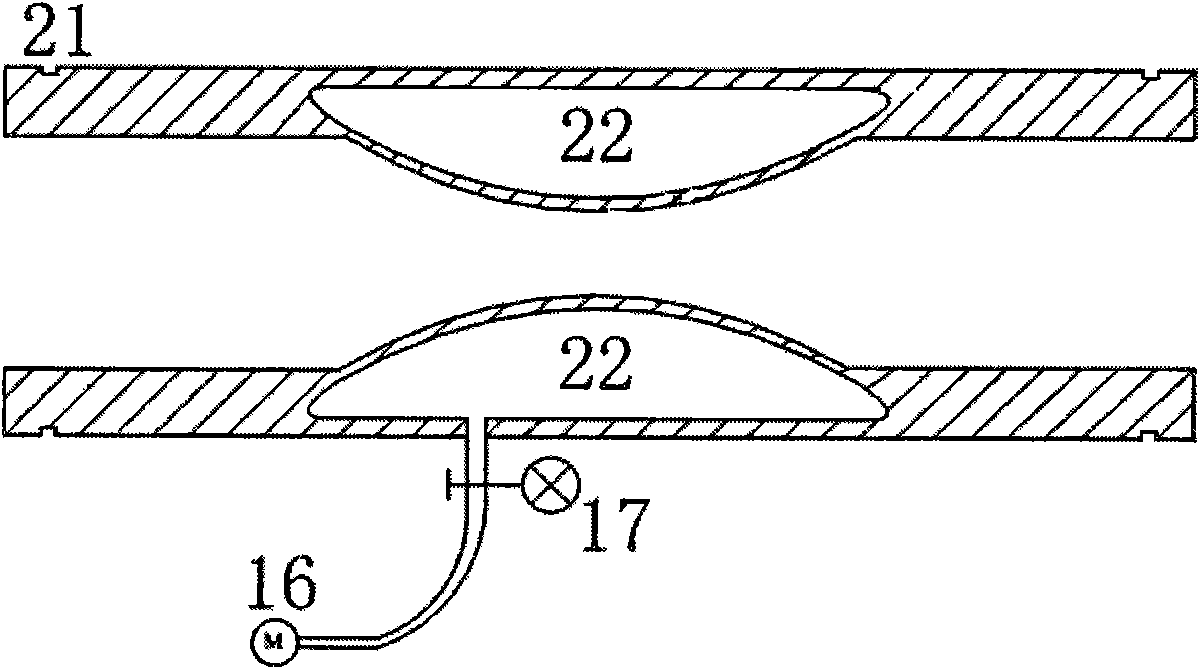
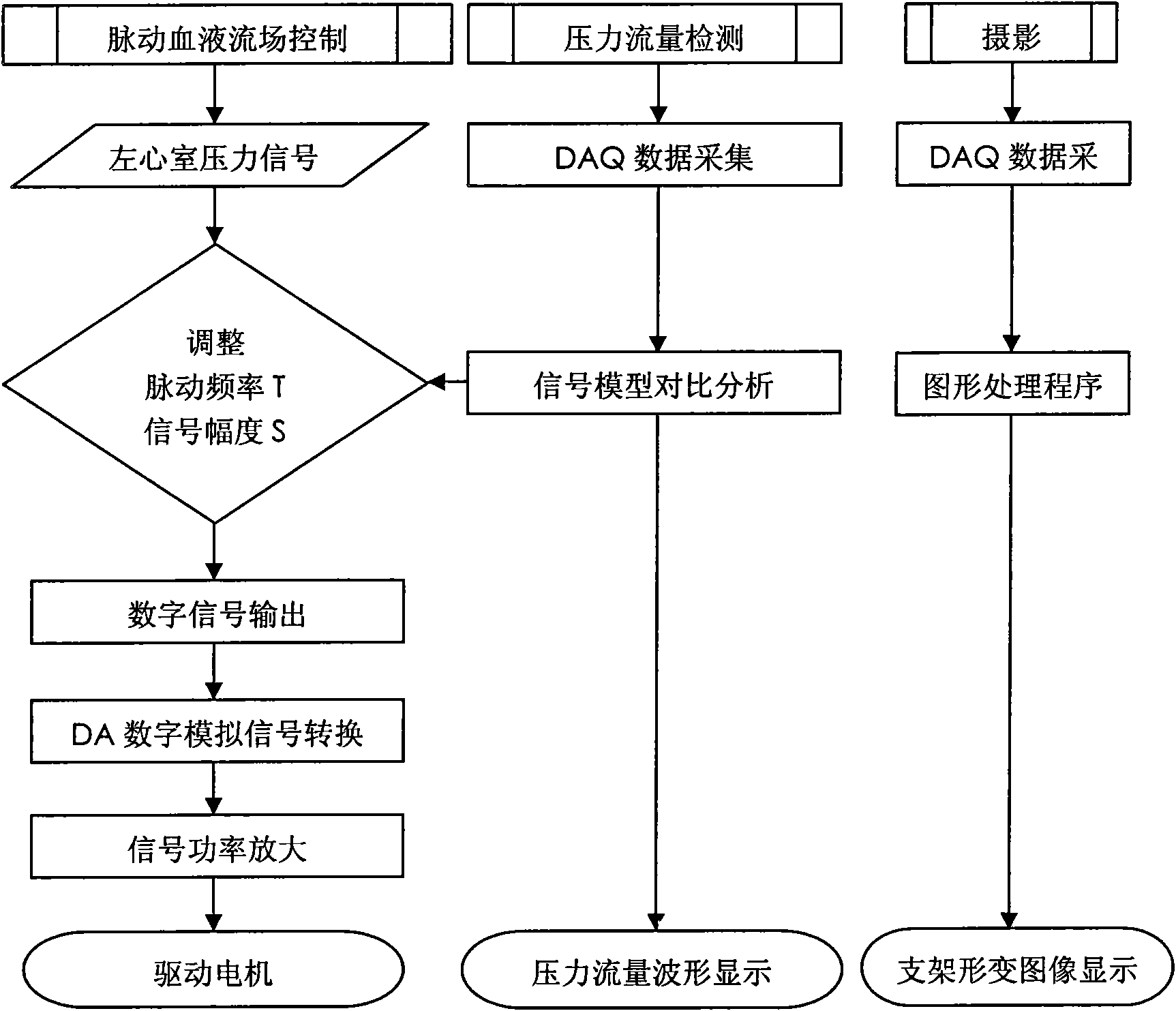
[0028] like figure 1As shown, the biomechanical experimental simulation device for intravascular stent implantation is composed of artificial cardiovascular system, artificial blood and pulsating blood flow field control system, and mechanical experimental observation device. The artificial cardiovascular system includes a closed fluid storage tank 5, a silicone left ventricle model 6, a silicone hollow sphere simulating the right atrium 7, an upper open fluid storage tank 8, an aortic arch model 10, an entering cranial artery model 11, and a descending aorta model 12 , a branch vessel model 14 and a narrow vessel module model 15; a silicone left ventricle model 6 that is geometrically similar to the left ventricle is soaked above the liquid storage tank 5, the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com