Medicine compounds for treating osteoporosis
A technology of osteoporosis and composition, which is applied in the direction of drug combination, drug delivery, bone diseases, etc., can solve the problem that the pathogenesis is not fully studied, and achieve remarkable curative effect, convenient drug use, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
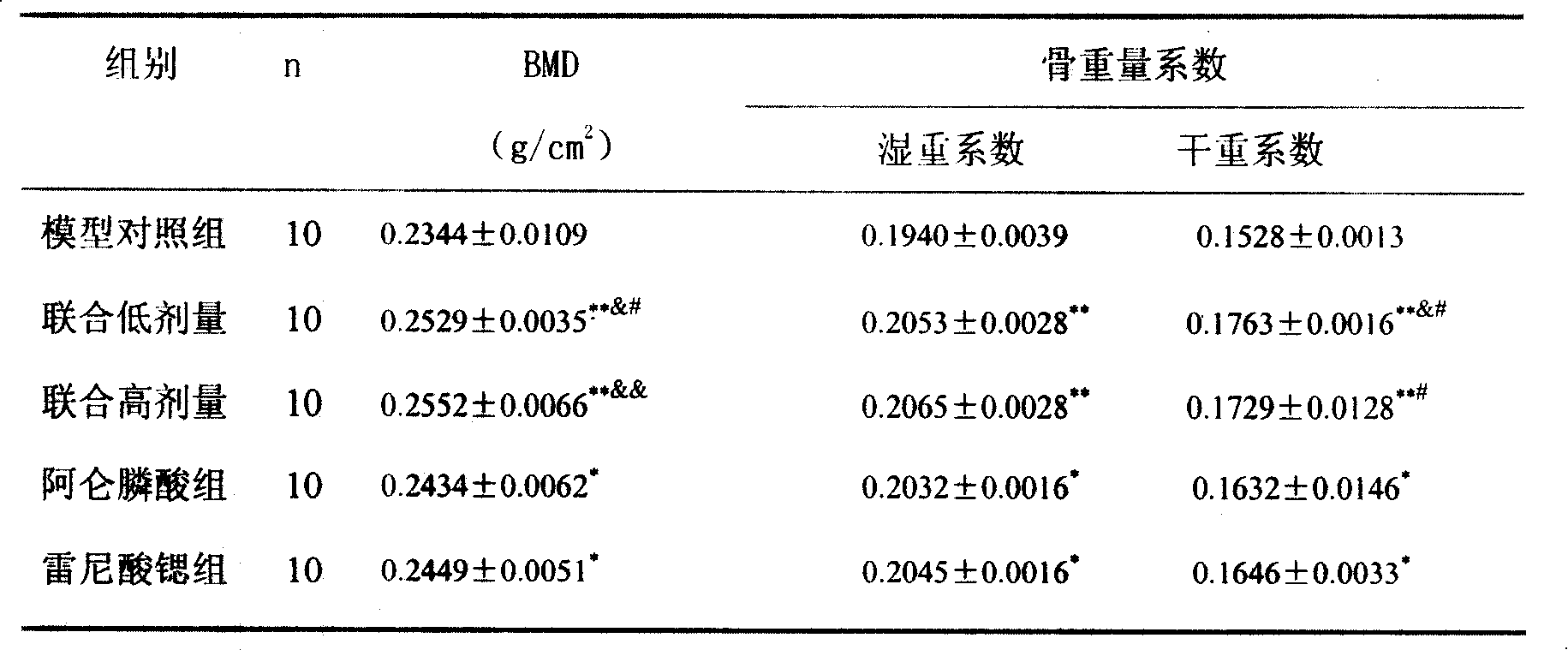
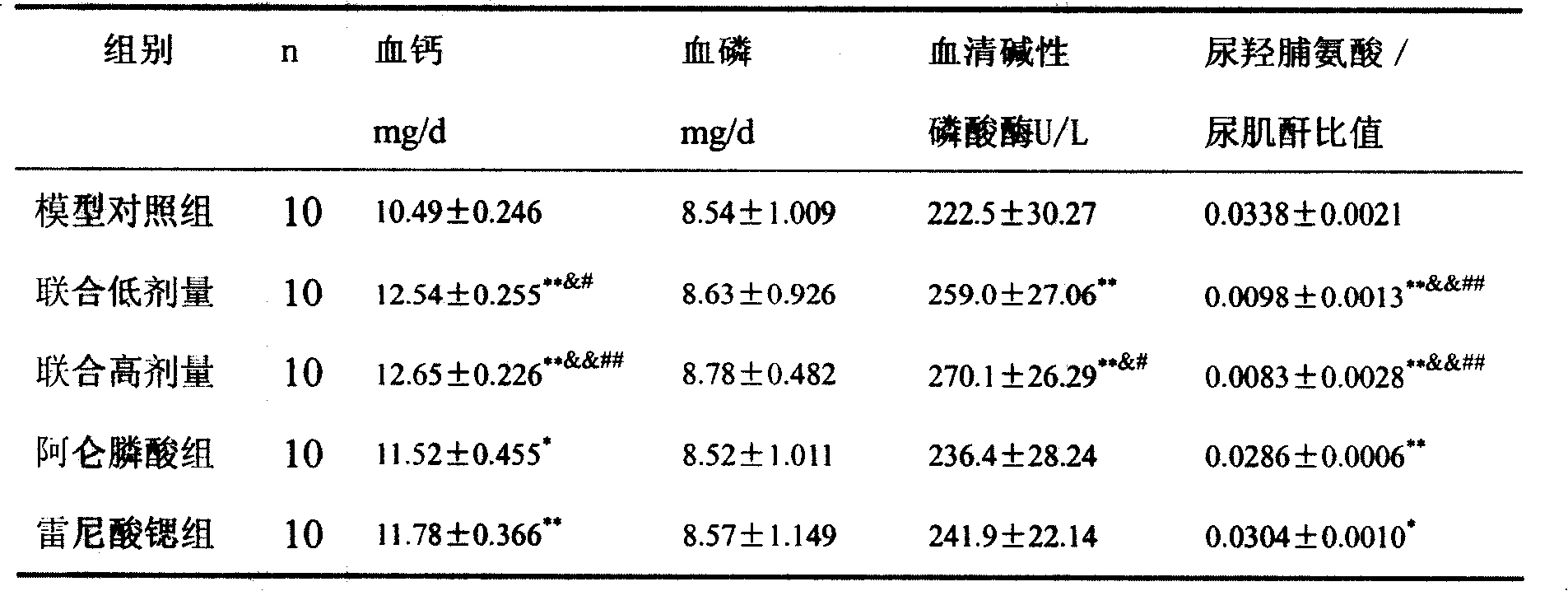
[0015] Example 1 Effects of strontium ranelate and alendronate sodium compound on castrated osteoporosis model rats
[0016] 1. Experimental materials and methods
[0017] 1) Experimental drugs
[0018] Alendronate Sodium, Strontium Ranelate.
[0019] 2) Experimental animals and modeling methods
[0020] 50 6-month-old female sterile SD rats (provided by Lunan Pharmaceutical Group New Times Pharmaceutical Animal Center, SPF grade).
[0021] Modeling method: firstly, rats were anesthetized with 100g / L chloral hydrate, injected intraperitoneally at 300mg / kg, and bluntly separated the abdominal muscle and retroperitoneum into the abdomen through a midline incision, found the ovary, ligated it with silk thread, and sutured the incision. The sutures were removed 1 week later.
[0022] 3) Grouping
[0023] random grouping;
[0024] Group A: model control group, 10 rats, the anesthesia method and operation path were the same as the modeling method above, ovariectomized, intraga...
Embodiment 2
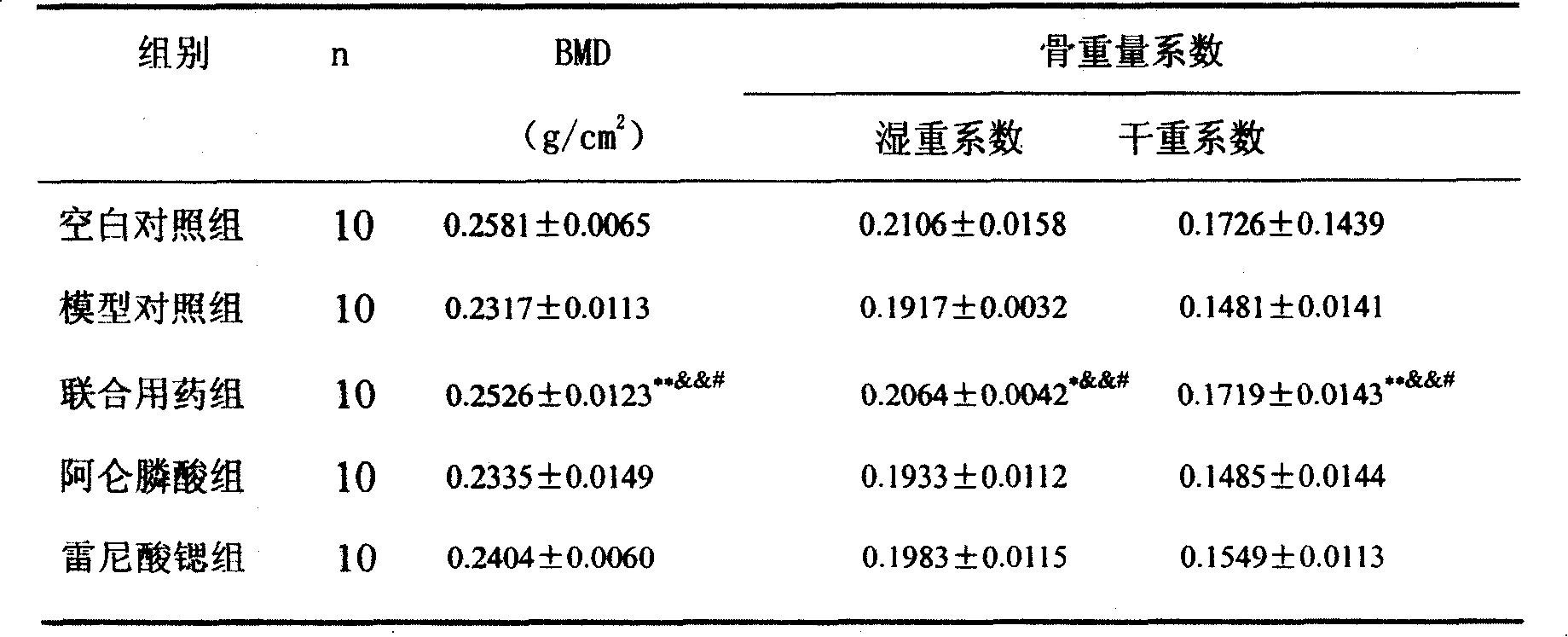
[0047] Example 2 Effects of strontium ranelate and alendronate sodium compound on osteoporosis rats modeled with retinoic acid
[0048] 1. Experimental method
[0049]Fifty 6-month-old female SD rats, weighing between 180 and 220 g, were selected from the Experimental Animal Center of Lunan Pharmaceutical Group and randomly divided into 5 groups:
[0050] Blank control group: 10 rats were given intragastric administration of 2ml of 1% sodium carboxymethylcellulose saline, once a day.
[0051] Retinoic acid-induced osteoporosis model group: 10 rats were intragastrically administered with 2ml of 4% retinoic acid suspension prepared by adding 1% carboxymethylcellulose sodium solution and retinoic acid, once a day.
[0052] Strontium ranelate and alendronate sodium compound group: 10 rats, as above modeling method, add strontium ranelate and alendronate sodium solution with 1% sodium carboxymethylcellulose solution to make a solution, press ranelate Strontium 700mg / kg + alendron...
Embodiment 3
[0071] The preparation of embodiment 3 strontium ranelate and alendronate sodium granules
[0072] Strontium ranelate 2000g
[0073] Alendronate Sodium 5g
[0074] Mannitol 2000g
[0075] Corn starch 2000g
[0076] Sucrose 8000g
[0077] Sodium Carboxymethyl Cellulose 800g
[0078] 10% starch slurry appropriate amount
[0079] Preparation Process:
[0080] Pass strontium ranelate, sodium alendronate, mannitol, cornstarch, sucrose, and sodium carboxymethylcellulose in the prescription through a 100-mesh sieve, weigh according to the prescription, mix well, and add 10% starch slurry to prepare To make soft material, granulate with 14 mesh sieve, dry at 70-80°C, granulate with 12 mesh sieve, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com