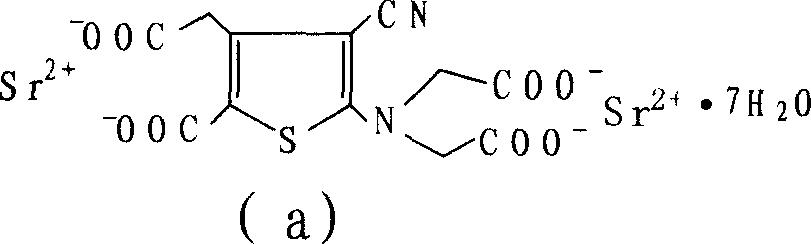
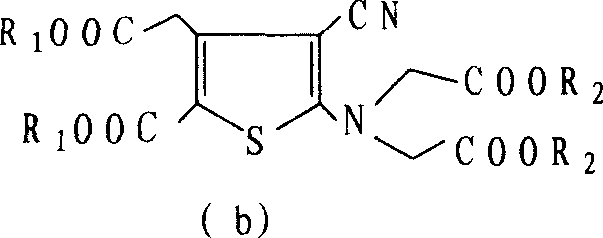
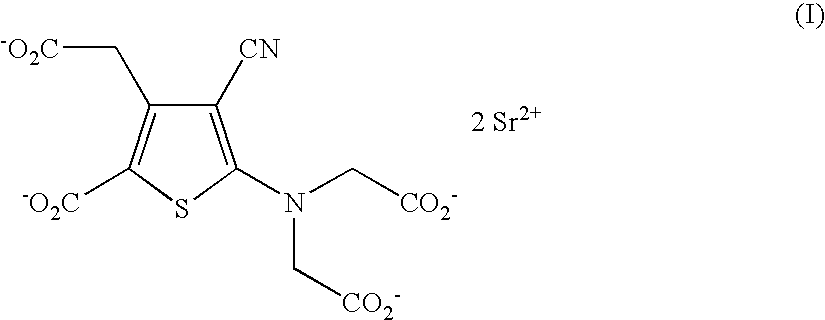
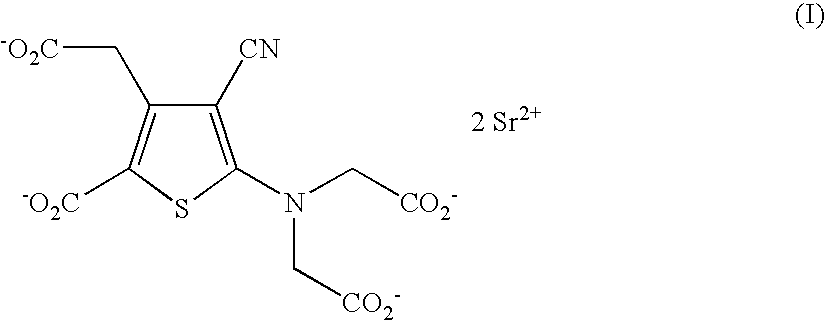
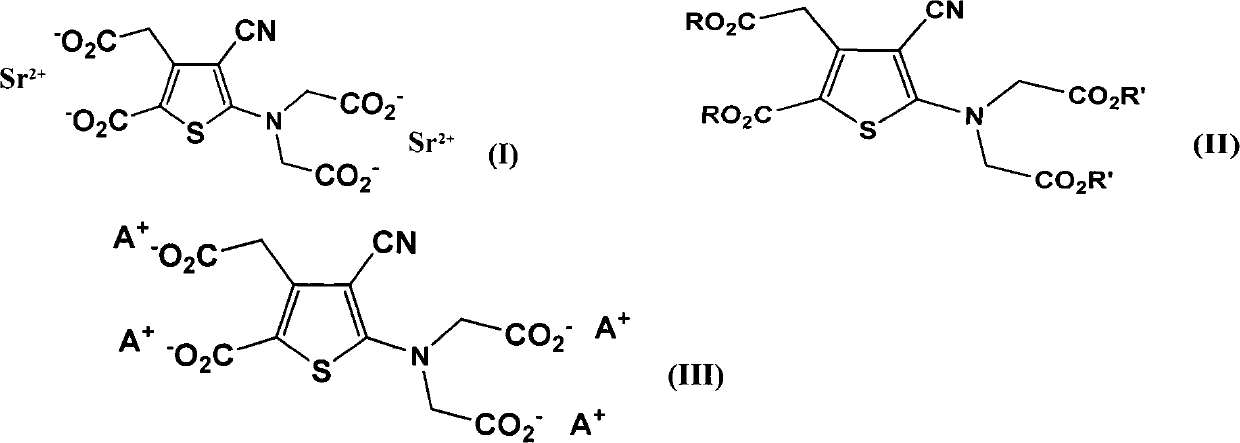
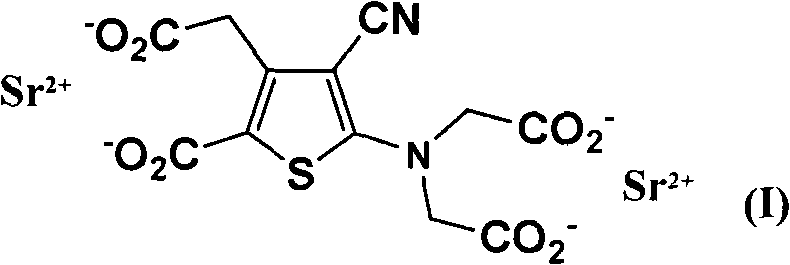
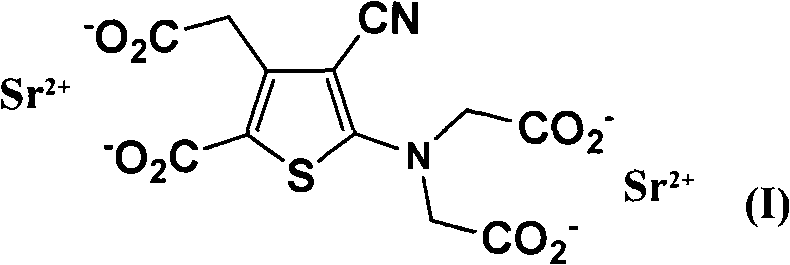
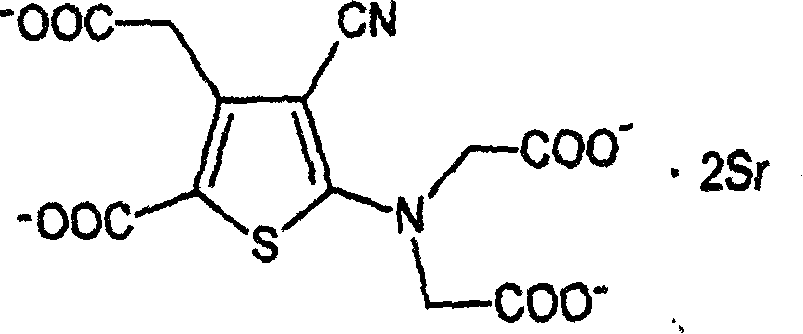
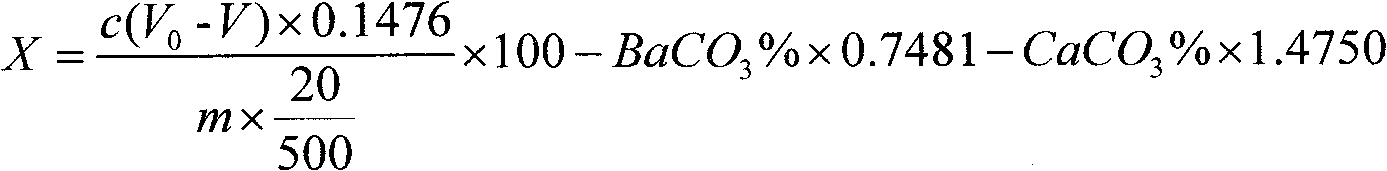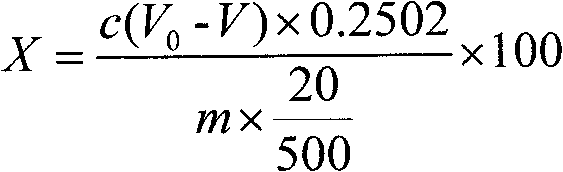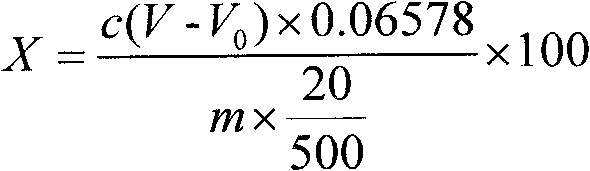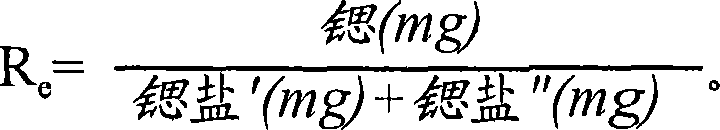Patents
Literature
101 results about "Strontium ranelate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
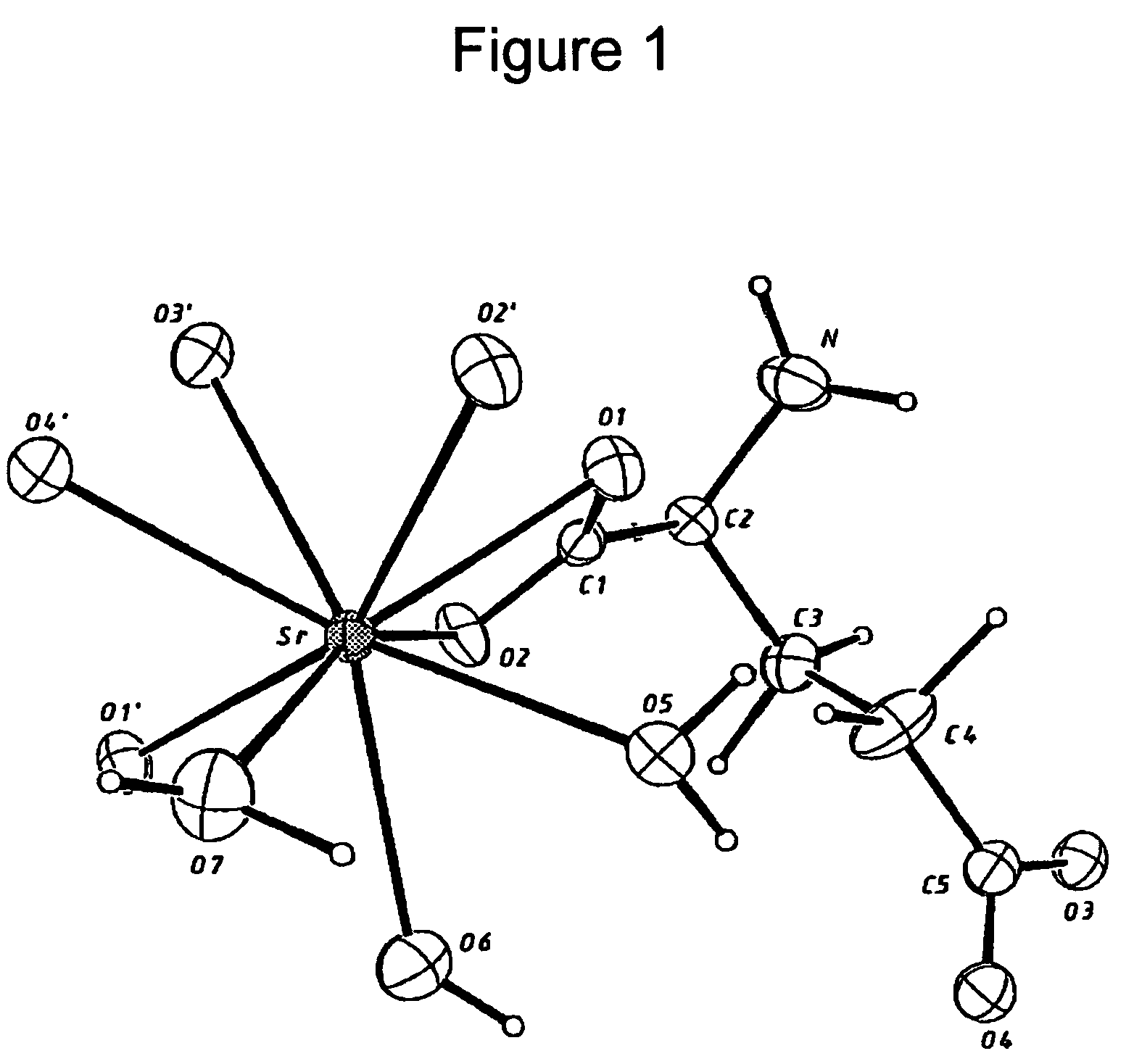
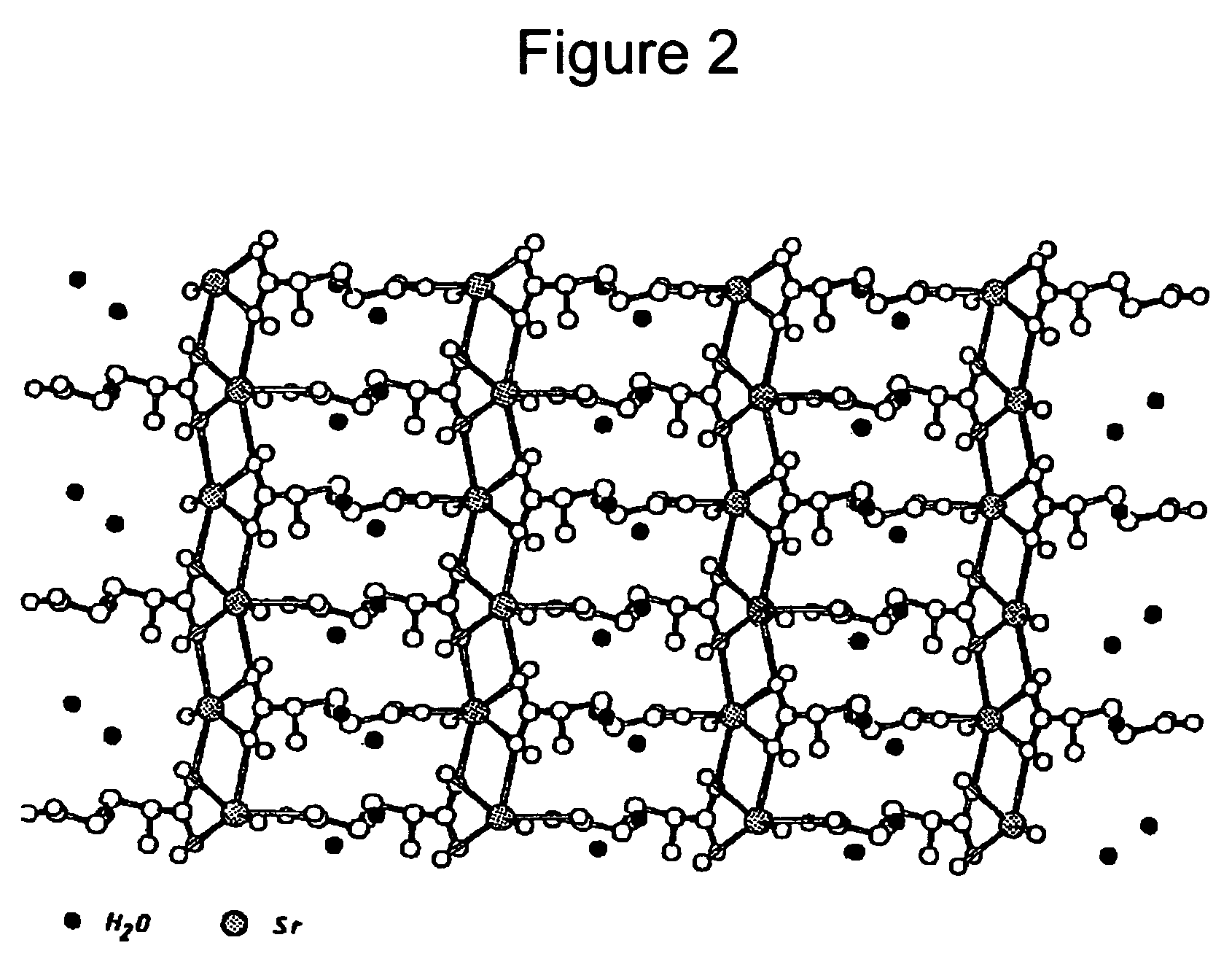
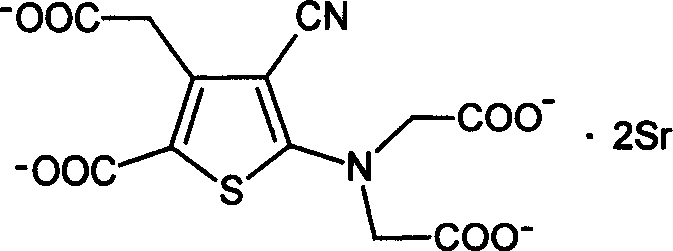
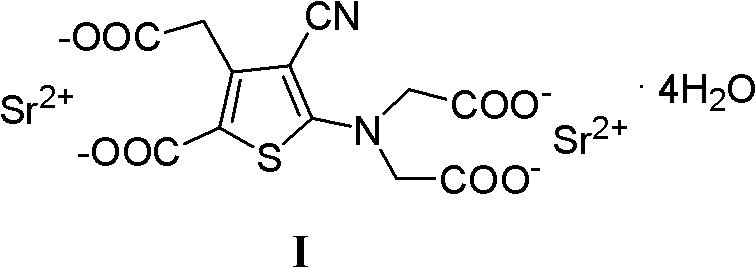
Strontium ranelate, a strontium(II) salt of ranelic acid, is a medication for osteoporosis marketed as Protelos or Protos by Servier. Studies indicate it can also slow the course of osteoarthritis of the knee. The drug is unusual in that it both increases deposition of new bone by osteoblasts and reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA).
Therapeutic oral composition
ActiveUS20130078197A1Alleviate hypersensitivityPromote remineralizationAntibacterial agentsCosmetic preparationsArgininePotassium
Disclosed are therapeutic oral compositions useful in the treatment of a variety of oral disorders, in which the composition can provide blockage of dentinal tubes, while at the same time provide antibacterial and anti-caries efficacy. The compositions include arginine in free or salt form, a mucoadhesive polymer, and at least one component selected from pyrophosphates, zinc salts, potassium salts, strontium salts, and mixtures thereof.
Owner:COLGATE PALMOLIVE CO
Process for the industrial synthesis of strontium ranelate and its hydrates
InactiveUS20040063972A1Organic active ingredientsOrganic chemistry methodsMedicinal chemistryStrontium ranelate
Owner:LES LAB SERVIER
Medicine compounds for treating osteoporosis
ActiveCN101229177AReduce dosageSignificant effectOrganic active ingredientsSkeletal disorderMicro structureSide effect
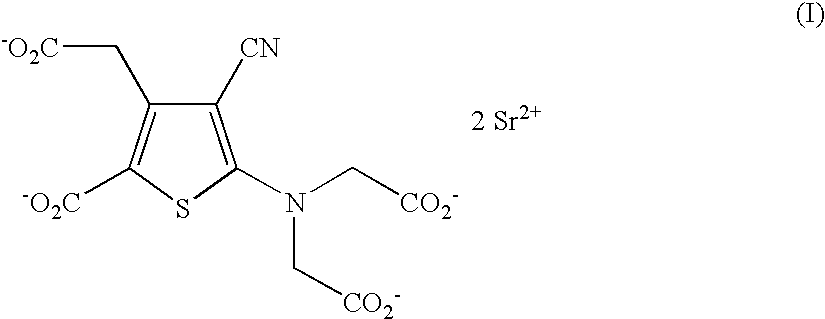
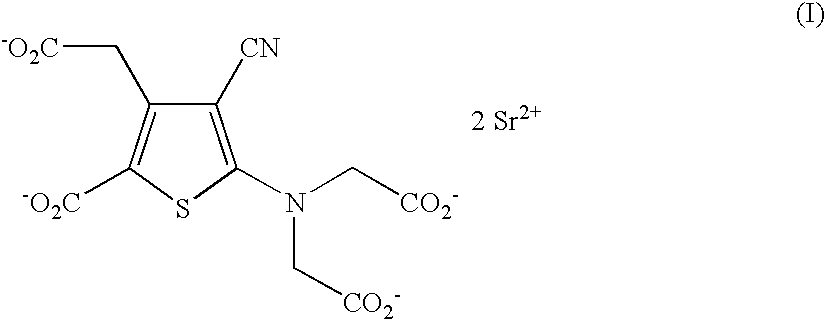
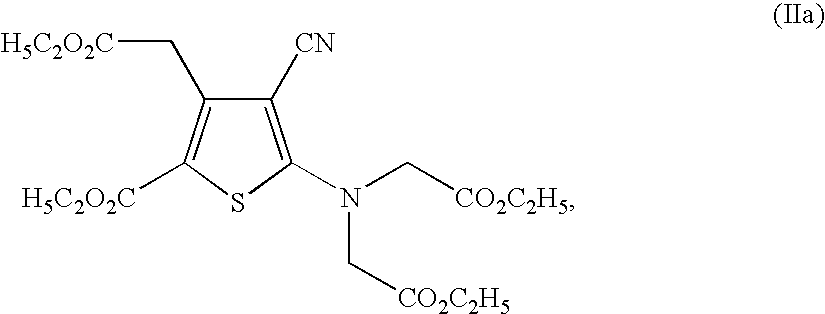
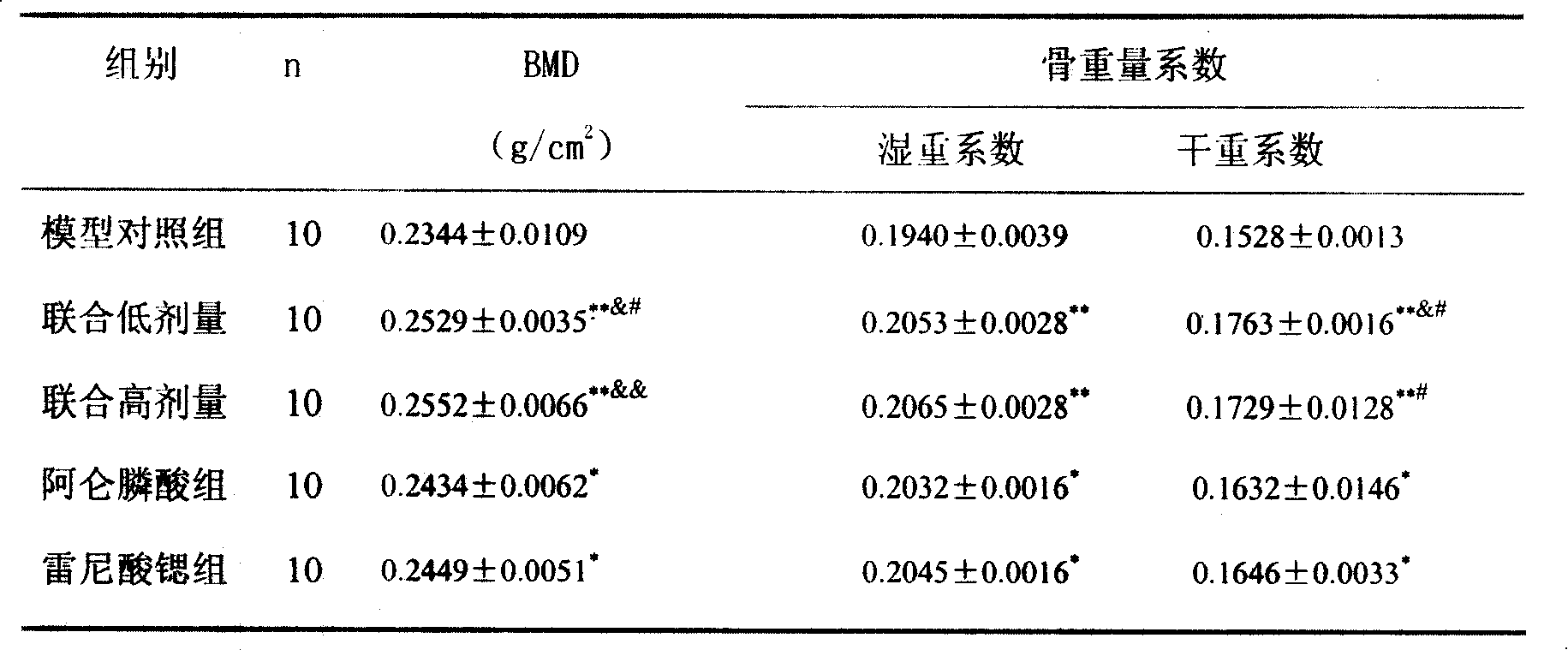
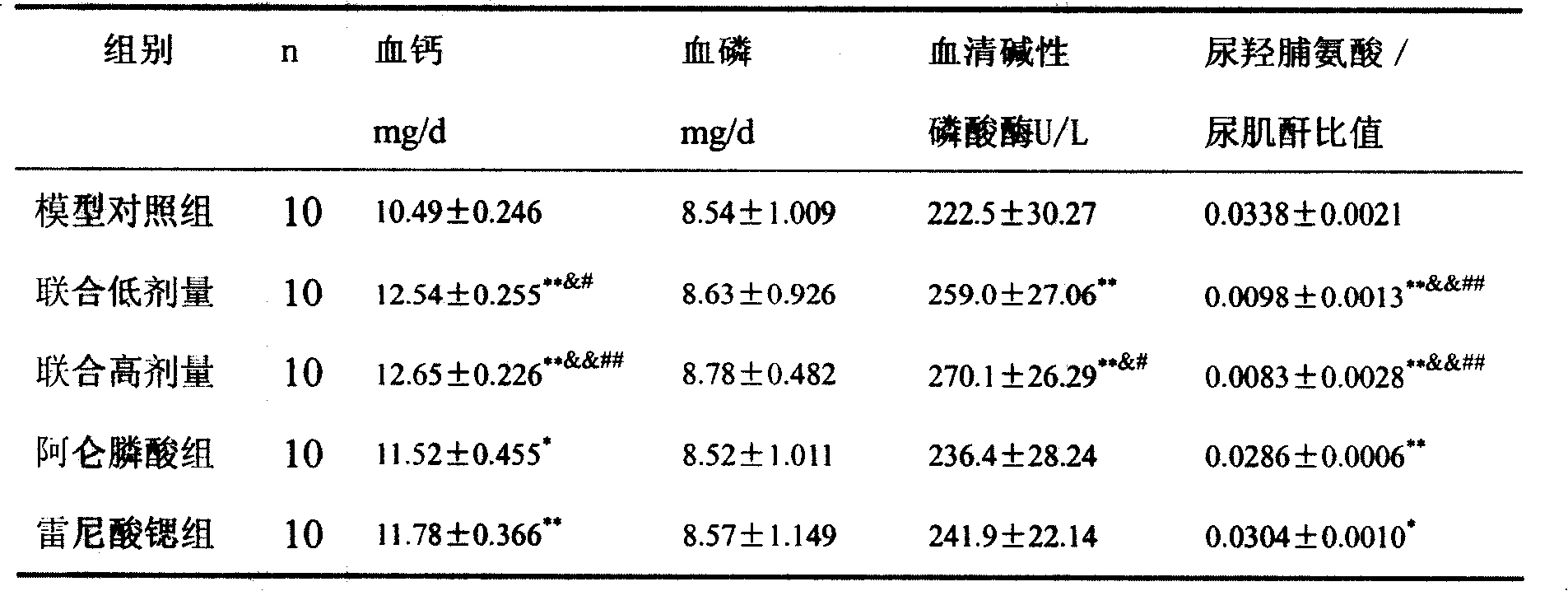
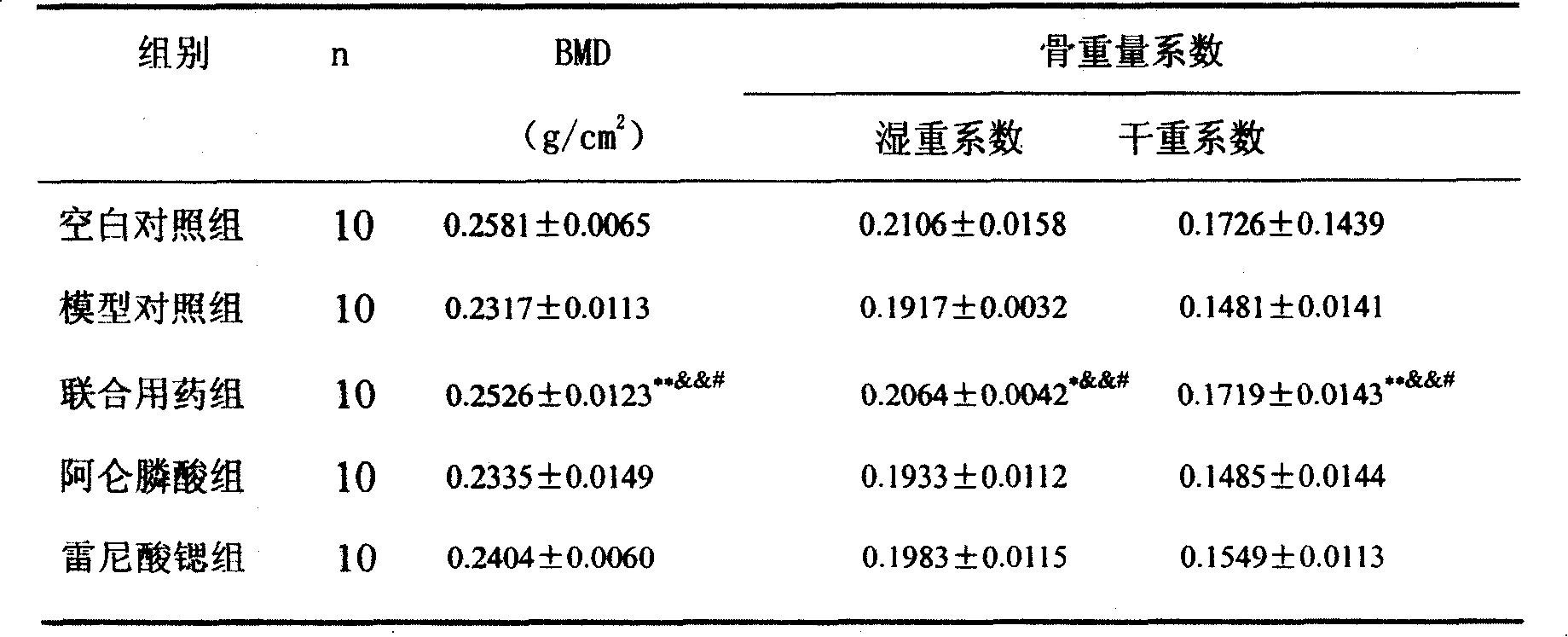
The invention provides a medical compound for treating osteoporosis which is characterized in that the invention contains strontium ranelate and bisphosphonate. Animal experiments indicate that the invention achieves the unexpected effect for treating the osteoporosis. The osteoporosis is a bone disease of the whole body characterized by the low bone mass and the degeneration of the micro structure of the bone organization, companying with the enhancement of the bone fragility and easy happened bone broken for which no ideal treatment medicine exists in the clinic. The bisphosphonate of the invention comprises alendronate, risedronate sodium, ibandronate, pamidronate, Etidronate, disodium clodronate and zoledronic acid, etc. In the invention, the dosage of the bisphosphonate is greatly reduced, which can effectively reduce the happening of side effects and is convenient to use the medicine.
Owner:LUNAN PHARMA GROUP CORPORATION
Water-soluble strontium salts for use in treatment of cartilage and/or bone conditions
Compounds and pharmaceutical compositions for use in the treatment and / or prophylaxis of cartilage and / or bone conditions and for methods of treating such condition. The compounds are salts of strontium that have a water-solubility of from about 1 g / l to about 100 g / l at room temperature, especially amino acid salts of strontium or dicarboxylic acid salts of strontium. Examples of novel water-soluble strontium salts are e.g. strontium glutamate and strontium alpha-ketoglutarate. The present invention also relates to an improved method for preparing the strontium salt of glutamic acid.
Owner:OSTEOLOGIX AS
Medicinal composition containing strontium salt
The invention relates to a medicinal composition containing strontium salts and vitamin D derivatives. Mixed with auxiliary materials acceptable on pharmacy, the medicinal composition of the invention can be prepared into oral formulations such as particulate granules, common tablets, chewable tablets, dispersible tablets, orally disintegrating tablets, effervescent tablets, buccal tablets, capsules, softgels, sustained release tablets, sustained release capsules, oral solutions, syrups, etc., and can be used to prevent and treat various primary or secondary osteoporosis.
Owner:FUKANGREN BIO PHARMA
Therapeutic oral composition
ActiveUS9579269B2Ameliorate dry mouthTreat erosion and gingivitisAntibacterial agentsCosmetic preparationsArginineStrontium
Disclosed are therapeutic oral compositions useful in the treatment of a variety of oral disorders, in which the composition can provide blockage of dentinal tubes, while at the same time provide antibacterial and anti-caries efficacy. The compositions include arginine in free or salt form, a mucoadhesive polymer, and at least one component selected from pyrophosphates, zinc salts, potassium salts, strontium salts, and mixtures thereof.
Owner:COLGATE PALMOLIVE CO
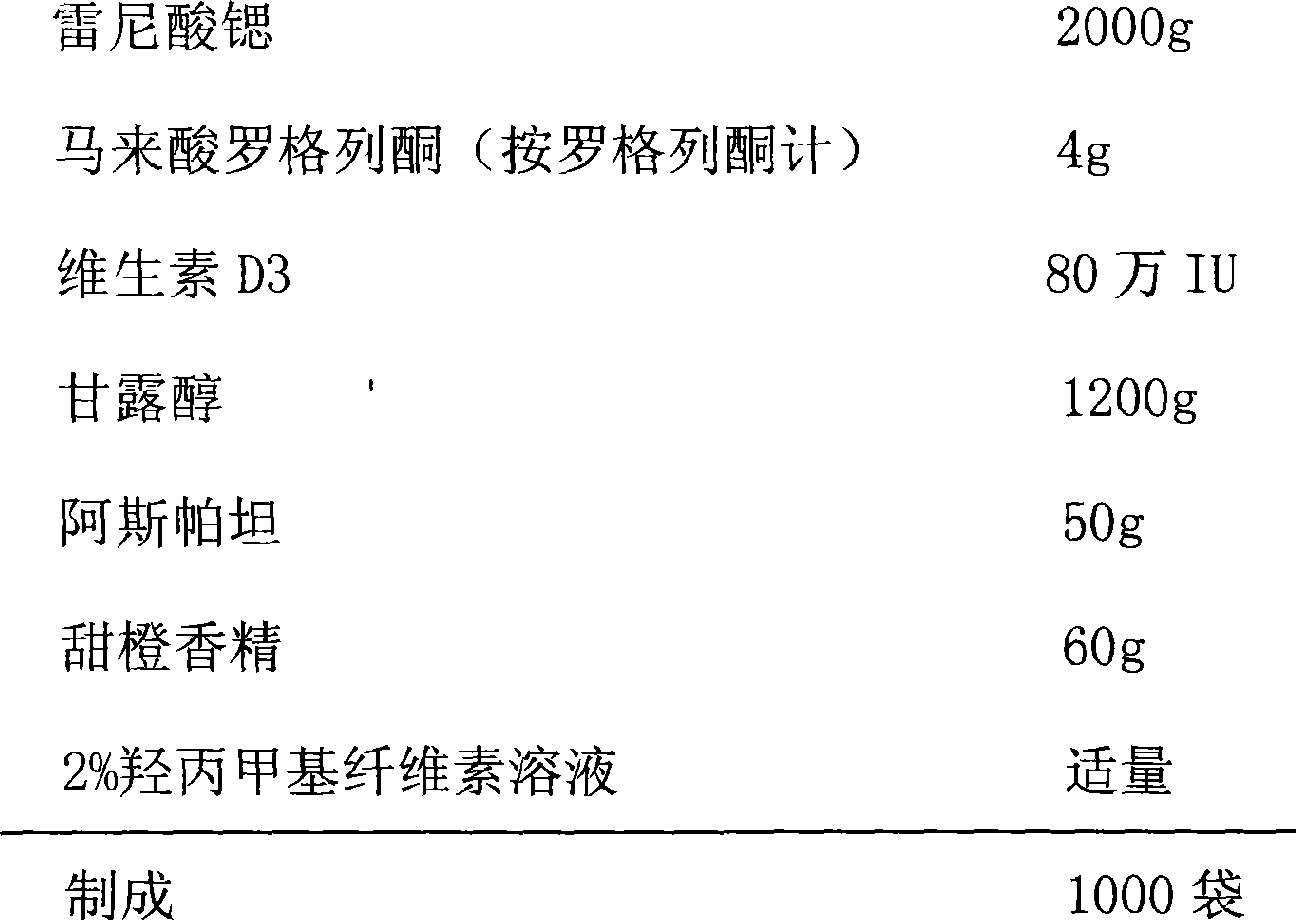
Medicinal composition containing strontium fuminate and vitamin D
InactiveCN1823764AMedication convenienceDoes not affect bioavailabilityPowder deliveryOrganic active ingredientsStrontiumPharmacology
A composite medicine and its packed composition for treating the osteoporosis of the woman after manopause contains strontium ranelate or its hydrate, VD and pharmacologically acceptable additives proportionally.
Owner:CHONGQING PHARMA RES INST +1
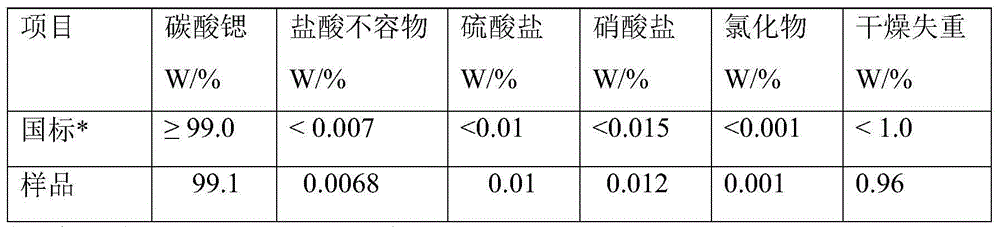
Method for determining strontium calcium barium in strontium carbonate
InactiveCN102419325AImprove stabilityDark colorMaterial analysis by observing effect on chemical indicatorCalcium EDTAStrontium ranelate
The invention relates to a method for determining strontium calcium barium in strontium carbonate. The method comprises the following steps: (1) preparing an ammonia-ammonium chloride buffer solution; (2) preparing and calibrating a 0.02mol / L EDTA standard solution; (3) preparing a 0.1% PAN indicator solution; (4) preparing a 0.02mol / L copper standard solution; (5) preparing an acetate-sodiumacetate buffer solution; (6) preparing and calibrating a potassium permanganate standard liquid; (7) preparing and calibrating a 0.01 mol / L sodium hyposulfite standard solution according to a routine method; (8) dissolving a sample, carrying out a dry filtering and collecting a filtrate; (9) carrying out capacity analysis of a. analysis and content calculation on strontium, b. analysis and content calculation on calcium, and c. analysis and content calculation on barium. The determination method of the invention is simply operated, has an obvious titration end, reduces time for determining strontium calcium barium in strontium carbonate and increases determination accuracy.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Compositions Comprising Strontium and Vitamin D and Uses Thereof
InactiveUS20080026037A1Convenient treatmentFew side-effectsHeavy metal active ingredientsBiocideStrontium carbonateStrontium chloride
The invention relates to the finding that very favorable pharmacokinetic characters are obtained by combining two strontium salts in one pharmaceutical composition. The present invention relates in one aspect to a pharmaceutical composition comprising at least two strontium salts for use as a medicament, and in particular for the treatment and prevention of bone disorders such as osteoporosis. The composition preferably comprises strontium carbonate and strontium chlorides. Further included may be a vitamin D compound, preferably vitamin D3.
Owner:MOKWALO SPF SA
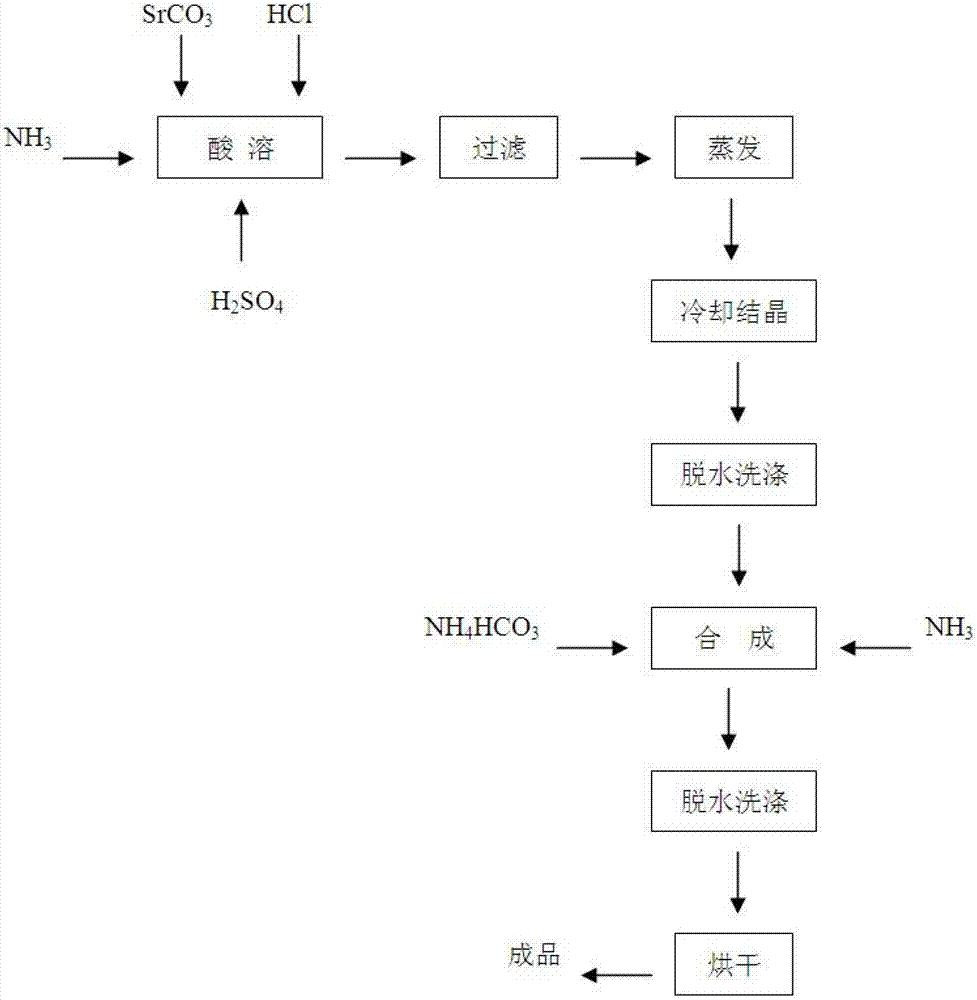
Novel method of producing strontium ranelate heptahydrate
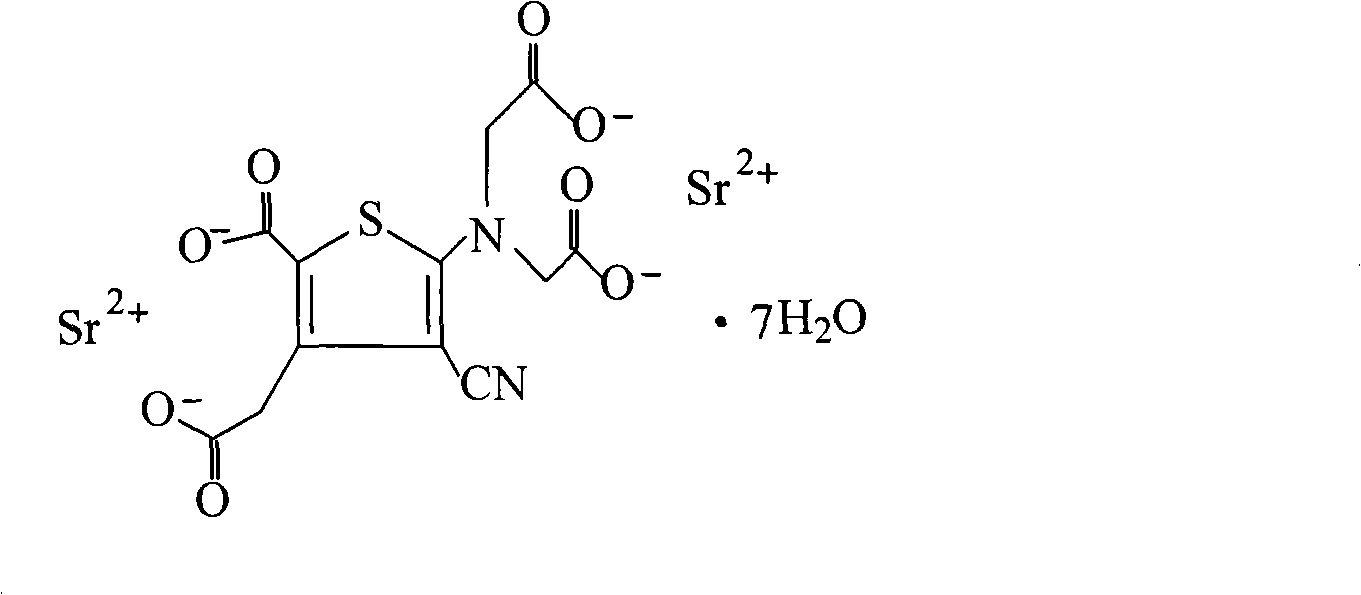
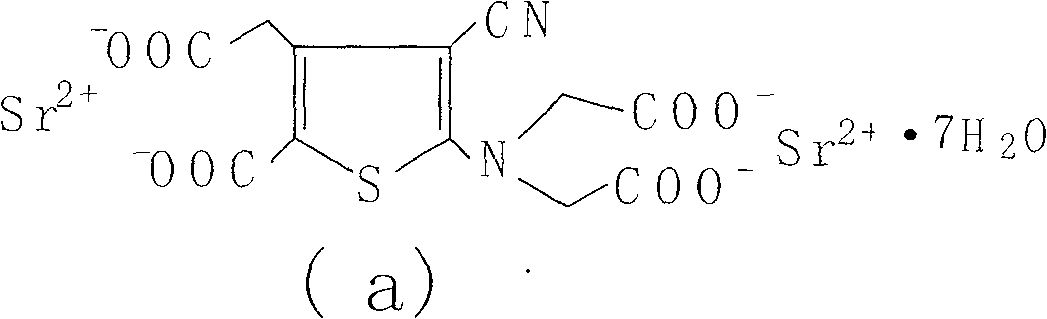
The invention relates to a new preparation method of strontium ranelate heptahydrate. The products produced by the method have water content of 19.0 per cent to 20.4 per cent and the relevant substances are lower than 0.5 per cent. The strontium ranelate heptahydrate produced by the method is stable under room temperature, has little water content differences between batches and is kept for a long time under natural environment with unchangeable moisture, content and relevant substances. The method is characterized in that: (1) the reflux of the 2-[N, N- 2(carboxymethyl)amino]-3-cyano-group-4-thiopheneacetic-5-carboxylic acid tetra ester (b) is carried out in the proper sodium hydroxide aqueous solution of alcohol for 5h to 6h and the pH value is kept over 10 during hydrolysis; (2) the ethanol of 20 per cent to 60 per cent is added and then the strontium chloride aqueous solution with 2times to 2.5times molal weight is directly added, precipitates the crystal, is filtered and dried; (3) water is added to carry out the reflux and the little salt packed in the crude product is removed, and the product is collected through heat filtration. The invention discloses the drug combination containing the strontium ranelate heptahydrate which is used for the treatment of osteoporosis of women after menopause.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Process for the industrial synthesis of strontium ranelate and its hydrates
InactiveUS7214805B2Organic active ingredientsOrganic chemistry methodsMedicinal chemistryStrontium ranelate
Owner:LES LAB SERVIER
Pharmaceutical combination with stable strontium ranelate and its preparations
InactiveCN101292977AGuaranteed stabilityChange structurePowder deliveryOrganic active ingredientsColor changesExcipient
The invention discloses a drug composite to promote the stability of strontium ranelate in the preparation. The invention is characterized in that after the effective dosage of the strontium ranelate for treatment is mixed with medicinal excipients, various dosage forms are produced by adding stabilizer. The dosage of the stabilizer refers to the dosage scope of excipients proscribed in the pharmacopoeia. The experiments and studies determine that the weight percentage of the stabilizer in the drug composite is 0.01-5 percent, which is preferred as 0.01-2 percent. The invention with a simple and unique way solves the color change problem of appearance of strontium ranelate or the problem of material increasing resulting from the excipients like citric acid, aspartame and saccharin sodium contained in the preparation; under the condition of not changing the structure of the strontium ranelate, a certain dosage of stabilizer is added into the excipients to achieve the purpose of the stability of the strontium ranelate. The method simplifies the process and saves the cost, and is more suitable for large-scale industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
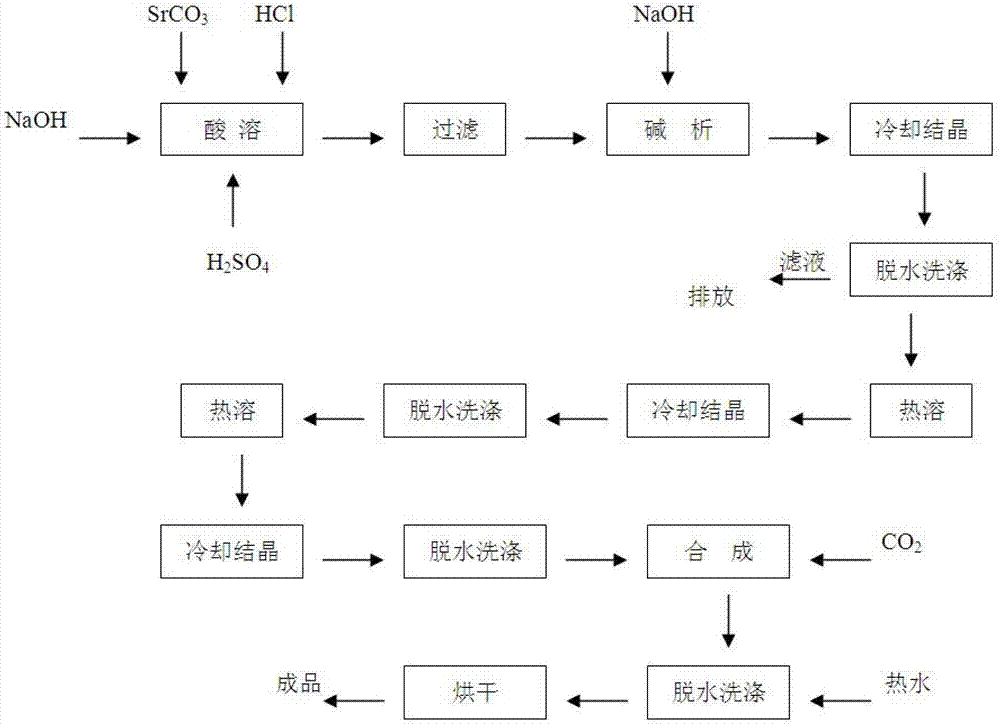
Preparation method of strontium carbonate with high purity
ActiveCN102765740AIncrease profitReduce consumptionCalcium/strontium/barium carbonatesCalcium/strontium/barium sulfatesStrontium ranelateIon
The invention discloses a preparation method of strontium carbonate with high purity. The method is characterized by comprising the following steps: (1) cooling and crystallizing a strontium sulfide solution, and separating out crude strontium hydroxide crystals; (2) recrystallizing the crude strontium hydroxide crystals after 2-5 times to obtain pure strontium hydroxide crystals; and (3) reacting the crystals prepared in the step (2) with liquid carbon dioxide to produce strontium carbonate, and dewatering and drying to obtain the strontium carbonate with high purity. According to the invention, brine from a leaching procedure in an original strontium carbonate production process is used as a raw material to realize low preparation cost; no sodium ion or chloride ion is added during the preparation process of the strontium carbonate with high purity; the product has easily controlled quality, and is simple for washing; no acid solution is added, and no waste gas is generated in the entire production process; wastewater generated in production process all returns to an industrial production line of strontium carbonate; therefore, the production process is green and clean.
Owner:NANJING JINYAN STRONTIUM IND
Method for preparing strontium ranelate
Owner:SHANDONG BOYUAN PHARM CO LTD
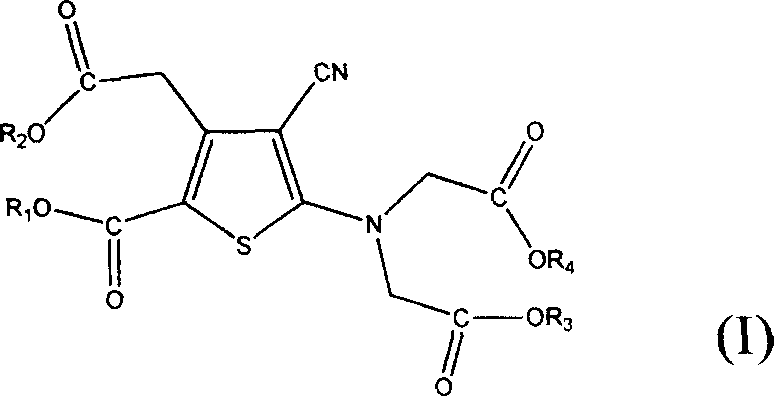
Novel method for preparing substituted thenoic acid ester and uses thereof
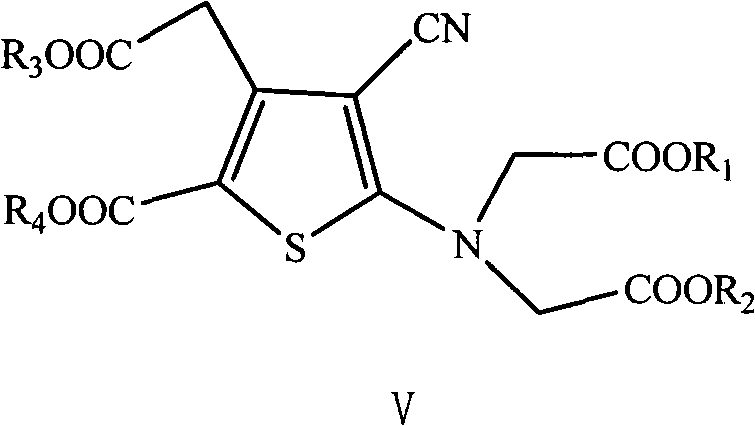
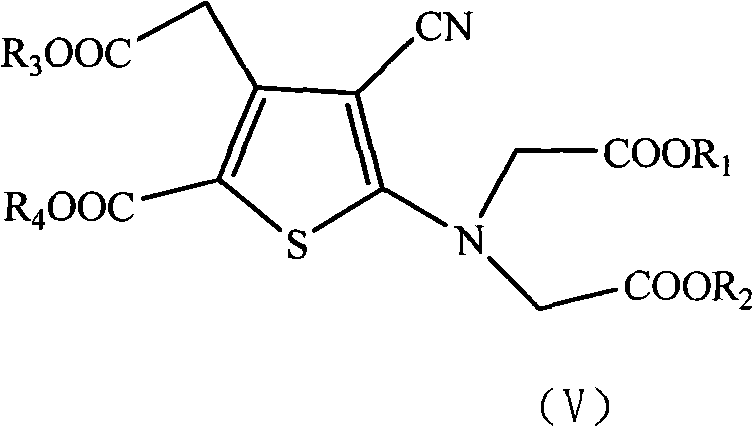
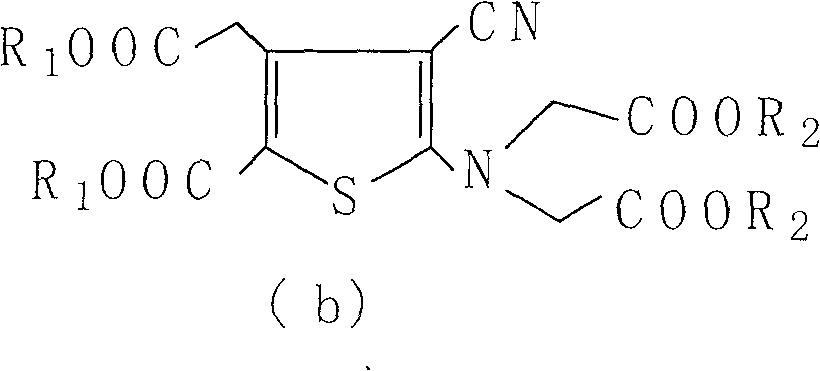
The present invention relates to a preparation method of the substituted thiophene formate as shown in formula (I), wherein the R1, R2, R3 and R4 can be the same and can be different; and the R1, R2, R3 and R4 respectively represent the alkyl of the straight chain or branched-chain of C1-C10, or the aryl of C6-C12, wherein the aryl can be replaced with 1 to 4 substituted bases. The present invention provides a novel method of preparing the substituted thiophene formate as shown in formula (I); the method is more economical and less harmful to the environment. The compound of the formula (I) produced in the present invention can be used for the synthesis of strontium ranelate.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Production method of granular anhydrous strontium chloride
ActiveCN102390855AReduce the temperatureLower the temperature; achieve the purpose of energy saving and environmental protectionCalcium/strontium/barium chloridesFluidized bedImpurity
The invention relates a production method of granular anhydrous strontium chloride. The method has the advantages of energy saving, environmental friendliness, simple process, convenience in operation and stable quality. The production method comprises the following steps: a. reacting strontium carbonate with hydrochloric acid to form strontium chloride solution; b. carrying out impurity removal and filtration treatment on the strontium chloride solution; c. evaporating and concentrating treated clear strontium chloride solution with drying hot tail air to increase concentration of the solution to 40-44 Baume degrees, and simultaneously absorbing anhydrous strontium chloride dust out of the hot tail air; and d. pumping the concentrated solution to a fluidized bed, spraying and granulating as well as simultaneously drying by the drying hot air of the fluidized bed, wherein the average particle size of the granulation product is controlled to be between 2 mm and 5 mm and the drying hot air at the equipment outlet can be used in the step c. Through verification, the energy consumption of the production method is 60% that of the existing technical scheme, the product has water content of 0.1-0.3% and the product can be stored for at least 6 months, and the corrosion to equipment is reduced.
Owner:CHONGQING YUANHE FINE CHEM
Process for preparing strontium ranelate tetrahydrate
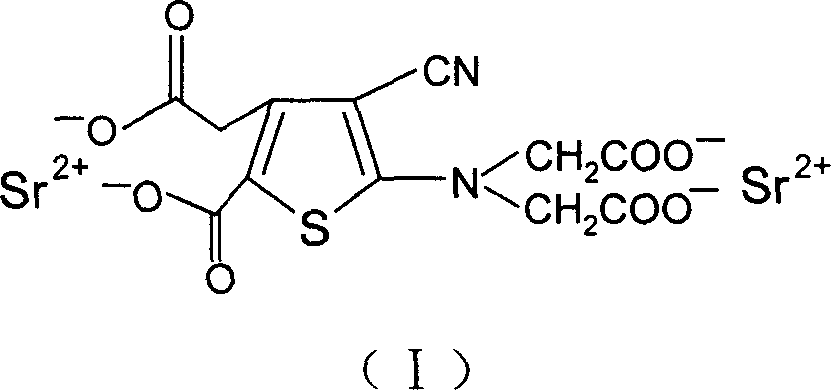
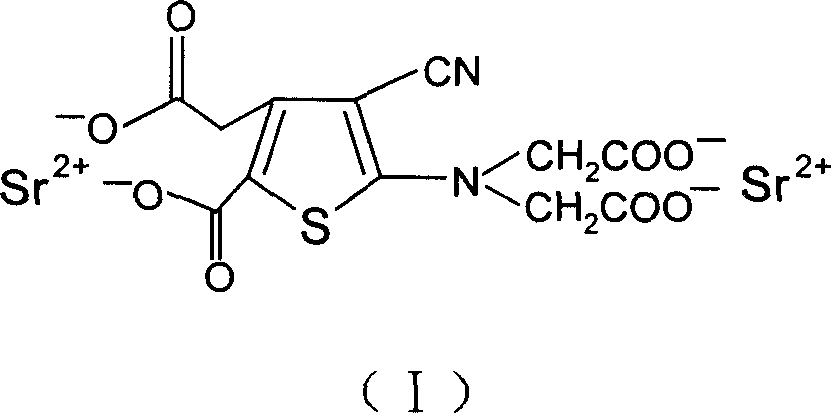
The invention discloses a making method of ranimycin strontium tetrahydrate, which comprises the following steps: adopting rough product of ranimycin strontium tetrahydrate with content over 80% as formula (I) as raw material; suspending in the water; adjusting pH value under indoor temperature or heating condition through acid until dissolving completely; obtaining the supernatant; washing through organic solvent; filtering; adjusting pH value through alkaline to neutral alkali-bias; stirring; filtering; drying to obtain the product with stable property.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Hair treatment agent with a polyvalent cation ii
The present disclosure relates to a hair treatment agent for reducing and / or avoiding bleeding out and / or fading of artificially produced hair colours with a pH in a range of from about 3.5 to about 5 containing—relative to the total quantity of hair treatment agent—a) from about 0.01 to about 10 wt. % of a polyvalent metal salt, wherein the metal salt is selected from the group of strontium salts, zirconium salts, hafnium salts, titanium salts, tin salts, aluminium salts, bismuth salts, lanthanum maleate, lanthanum chloride and mixtures thereof andb) from about 0.01 to about 10 wt. % of an organic acid selected from the group of tartaric acid, citric acid, maleic acid, fumaric acid, salicylic acid, lactic acid, malic acid, amino acids and mixtures thereof.
Owner:HENKEL KGAA
Method for preparing strontium ranelate
ActiveCN101775002ALow impurity contentEasy to operateOrganic chemistryOrganic solventLithium hydroxide
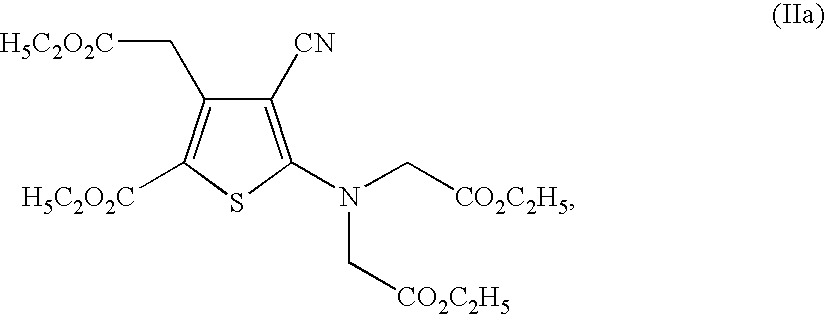
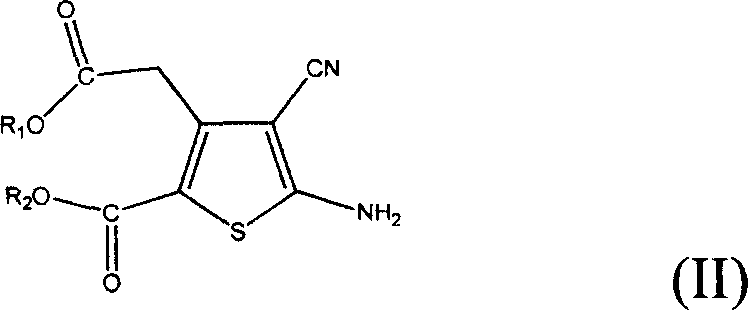
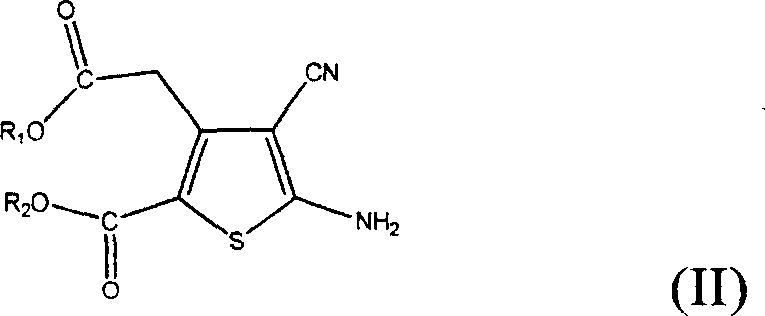
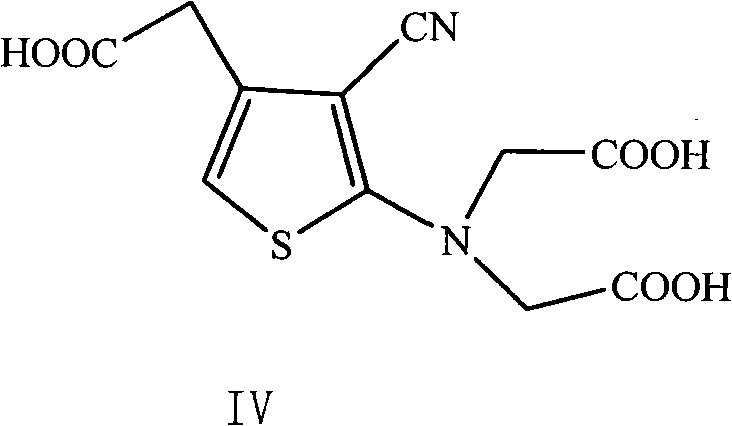
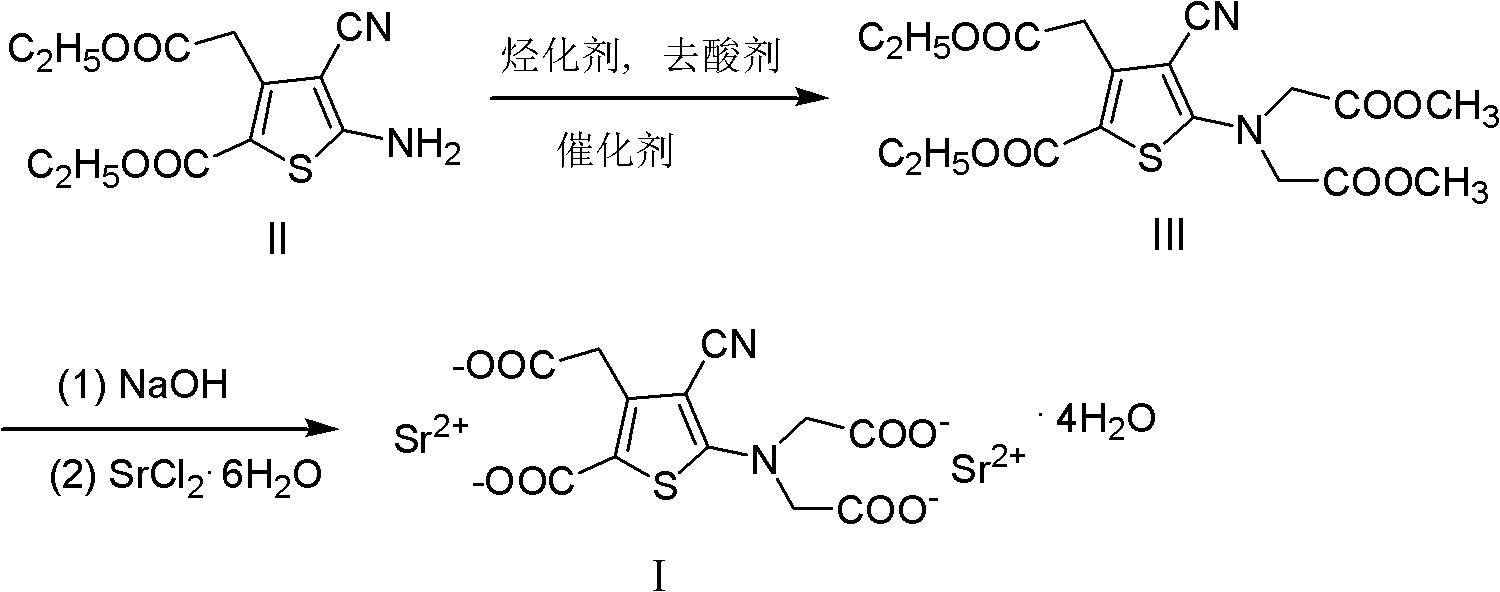
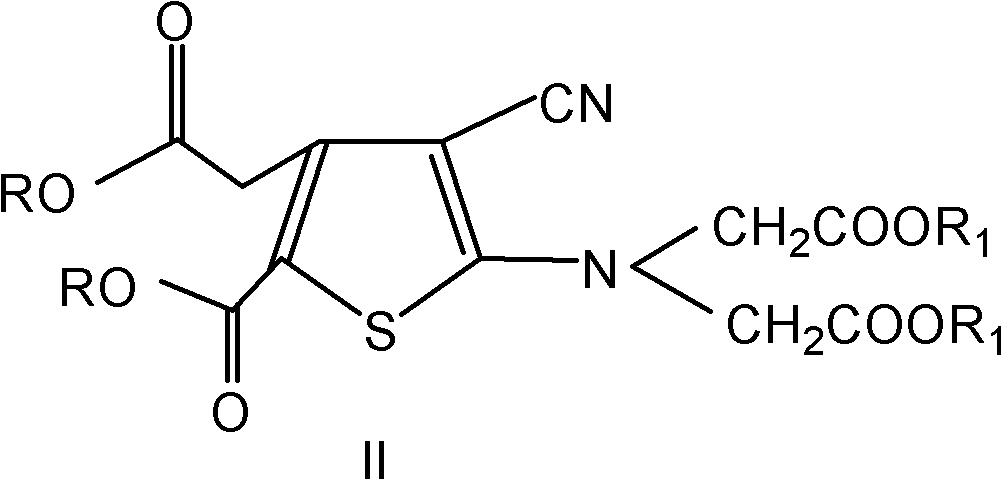
The invention provides a method for preparing strontium ranelate (I). The method is characterized in that: in a mixed liquor of water and an organic solvent, the compound in the formula (II) reacts with sodium hydroxide, potassium hydroxide or lithium hydroxide to obtain a compound in the formula (III); then the compound in the formula (III) is distilled to remove the organic solvent to obtain aqueous solution of the compound in the formula (III); and the obtained product reacts with strontium chloride in the aqueous solution, and filtering and separating the reaction solution to obtain strontium ranelate, wherein R and R' can be the same or different and respectively respects C1 to C6 linear chain or branched chain alkyl; and A respectively represents Na, K or Li. The strontium ranelate prepared by the method provided by the invention has obviously reduced single impurity content.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Strontium ranelate dry suspension
The invention discloses a dry suspension containing strontium ranelate. The dry suspension contains the following components by weight ratio: 50-90 per cent of lactose, or xylitol, or mannite or 50-90 per cent of the combination of lactose, xylitol, mannite; 10-49 per cent of strontium ranelate; 0.1-10 per cent of correctives; 0.1-10 per cent of colorant; and 1-20 per cent of adhesive. With good stability, the dry suspension containing strontium ranelate disclosed in the invention is used for curing and preventing the menopausal osteoporosis.
Owner:BEIJING D VENTUREPHARM TECH DEV
Double antiallergic toothpaste containing paeonol and strontium salt
InactiveCN108030706ASignificant anti-allergic effectSignificant analgesic desensitization effectCosmetic preparationsNervous disorderPreservativeStrontium
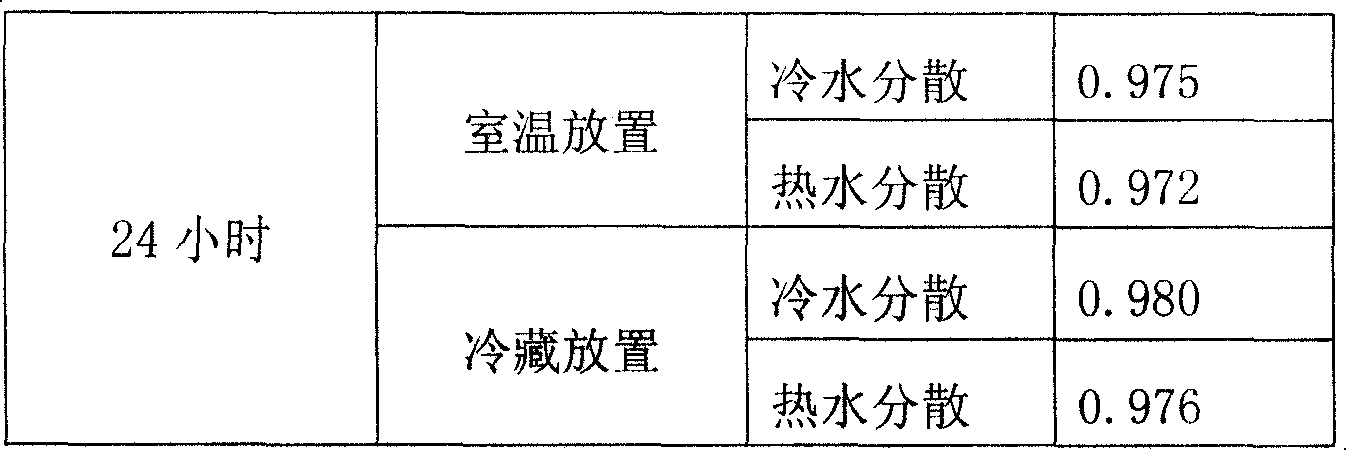
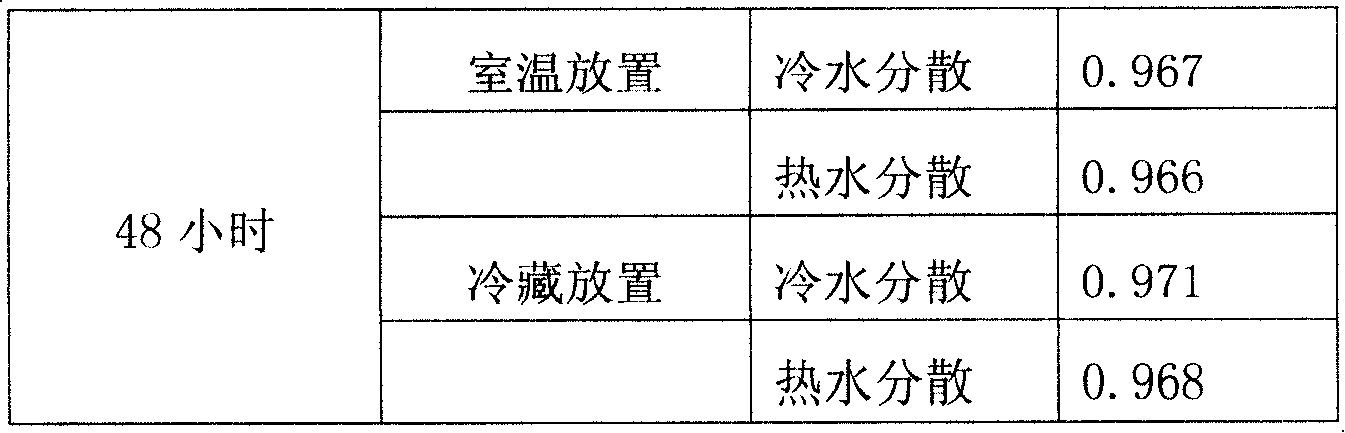
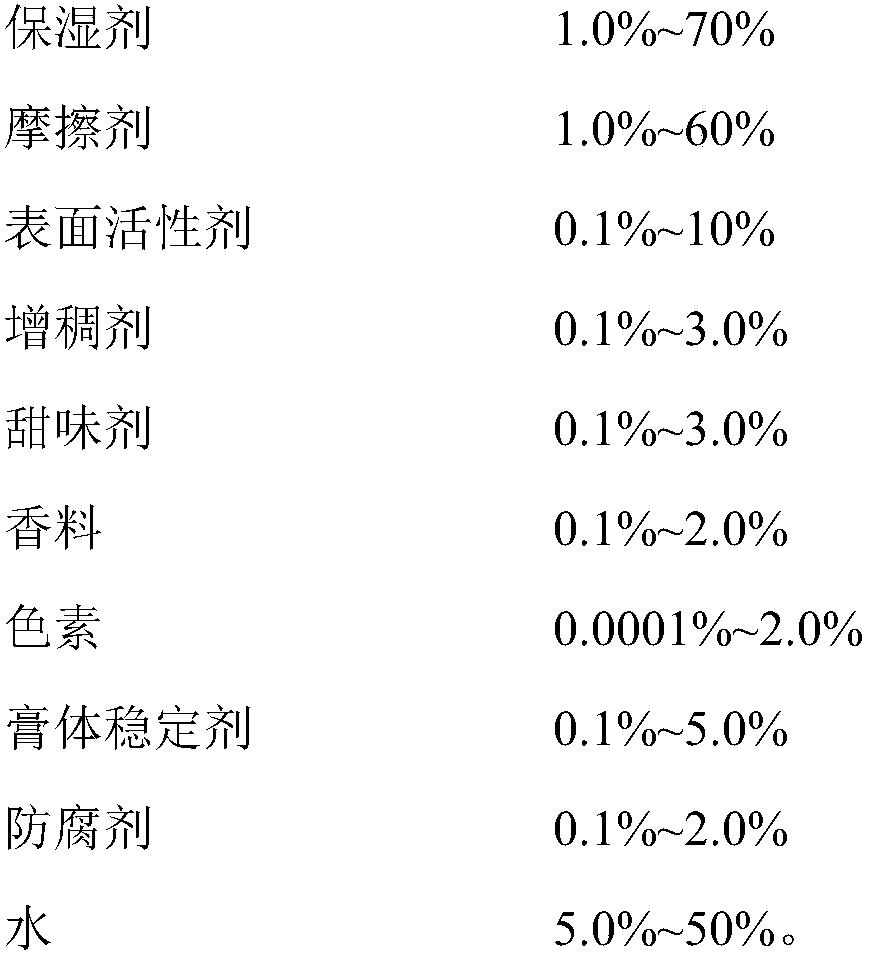
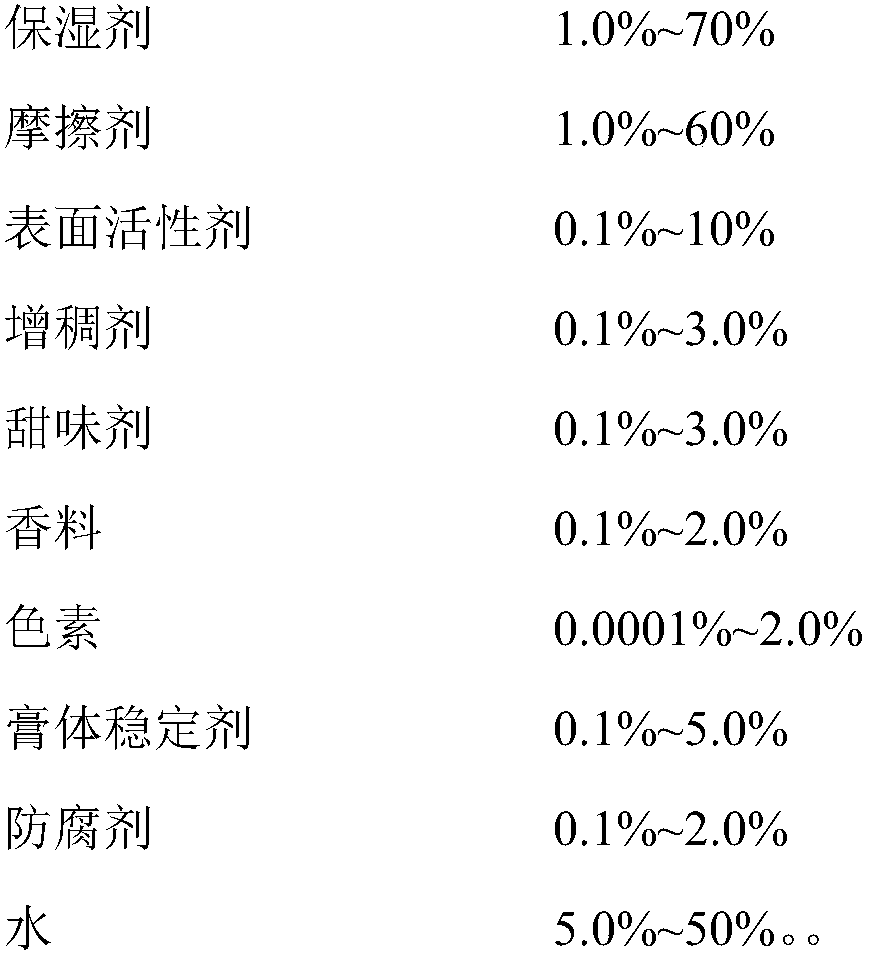
The invention provides double antiallergic toothpaste containing paeonol and strontium salt. The double antiallergic toothpaste is prepared from an antiallergic component and a base material component, wherein the antiallergic component is prepared from 1.0 to 3.0 percent by weight of strontium salt and 0.2 to 1.0 percent by weight of paeonol; the base material component is prepared from a humectant, an abrasive, a surfactant, a thickening agent, a sweetening agent, perfume, pigment, a paste stabilizer, a preservative and water. The paeonol and the strontium salt are added into a toothpaste base material in a specific mixture ratio in a supplemental way, and act on dental nerve in a cooperative manner, so that the double antiallergic toothpaste has a remarkable antiallergic effect.
Owner:上海美加净日化有限公司
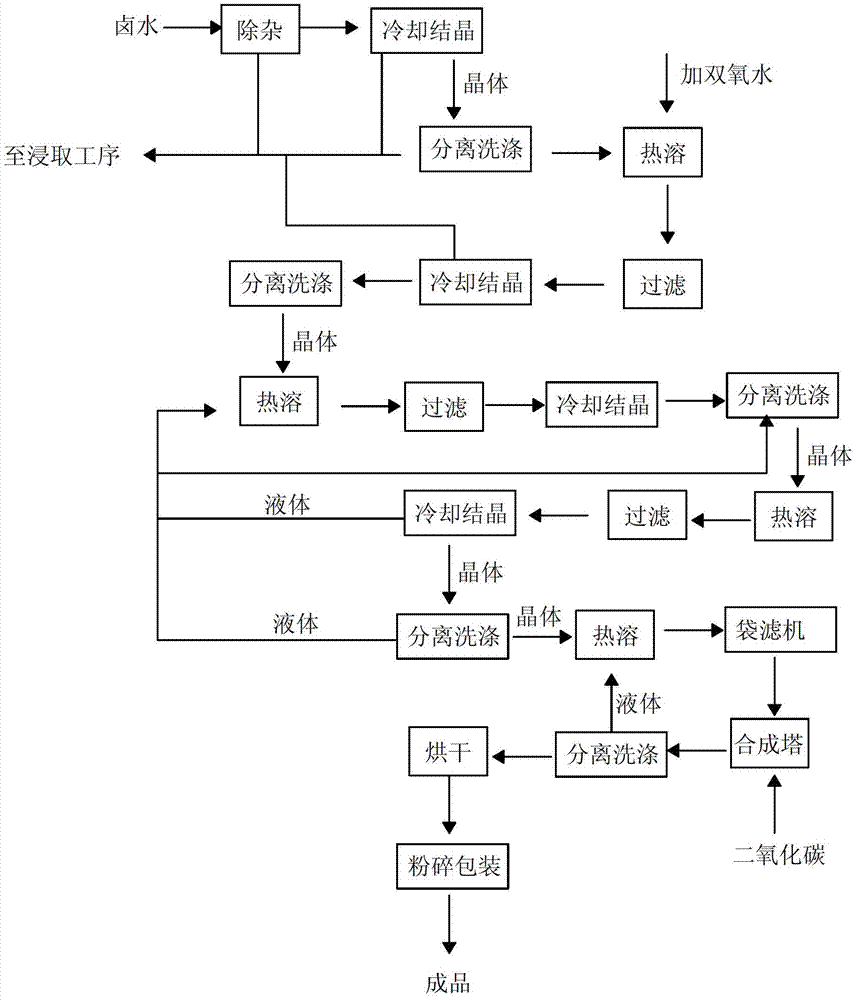
Microbial preparation method of strontium carbonate
InactiveCN104313055AEasy to trainCultivate continuous expansionMicroorganism based processesFermentationSodium bicarbonateSodium lactate
The invention discloses a microbial preparation method of strontium carbonate. The method comprises the following steps: adjusting pH value of a FeCl3 solution to 10-12 by the use of a NaOH aqueous solution at normal temperature by a titration method so as to carry out a precipitation reaction, centrifuging a mixed liquor, removing a supernatant, washing a precipitate with deionized water, and centrifuging to obtain hydrated iron oxide; preparing a reaction liquid containing strontium nitrate, sodium bicarbonate, sodium lactate and hydrated iron oxide by using water as a solvent, inoculating a culture solution which undergoes enlarge culture and contains iron-reducing bacteria to the above reaction liquid, and carrying out an anaerobic reaction at 20-50 DEG C for 2-5d to obtain a solution containing a strontium carbonate precipitate; standing for 1h, filtering, removing a supernatant, washing a white precipitate successively by using distilled water and absolute ethyl alcohol in turns, and drying to prepare a high-purity strontium carbonate powder. By wet-process one-step synthesis, the strontium carbonate powder in different granular sizes and shapes can be prepared, satisfying different purposes.
Owner:NANJING UNIV OF TECH
Pharmaceutical composition containing PPAR regulator and strontium salt
Composition preparation or pharmaceutical composition related to the invention contains (a) PPAR regulator, (b), strontium salt as active component, which is or is not combined with one or more acceptable excipients in pharmacy, the active component being used at the same, in a separated way or in sequence; the composition is used to improve, prevent or treat osteoporosis caused by prevention or treatment of PPAR regulator or diseases prevented or treated by PPAR regulator and groups with osteoporosis.
Owner:CHONGQING MEDICAL UNIVERSITY
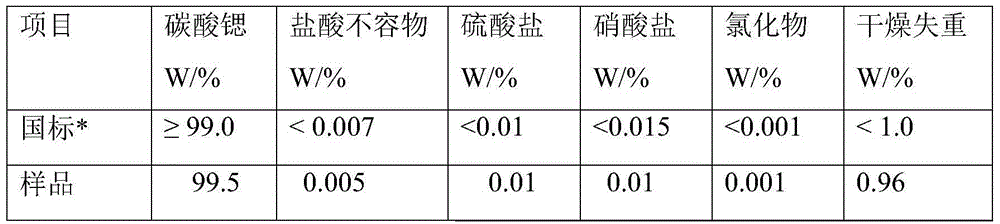
High-purity strontium ranelate and preparation method thereof
The invention relates to high-purity strontium ranelate and a preparation method thereof. The high-purity strontium ranelate is characterized in that the purity of the strontium ranelate is not lower than 99.5%, and a single impurity is not greater than 0.4%. The high-purity strontium ranelate is suitable for medicinal use and can effectively control the quality of products. The invention also relates to a medicine composition prepared by mixing a medicinal carrier and the high-purity strontium ranelate.
Owner:CHONGQING PHARMA RES INST
Compositions comprising strontium and vitamin d and uses thereof
ActiveCN101018586AHeavy metal active ingredientsSkeletal disorderStrontium carbonateStrontium chloride
The invention relates to the finding that very favorable pharmacokinetic characters are obtained by combining two strontium salts in one pharmaceutical composition. The present invention relates in one aspect to a pharmaceutical composition comprising at least two strontium salts for use as a medicament, and in particular for the treatment and prevention of bone disorders such as osteoporosis. The composition preferably comprises strontium carbonate and strontium chlorides. Further included may be a vitamin D compound, preferably vitamin D3.
Owner:MOKWALO SPF SA
Preparation method of strontium ranelate
The invention relates to the field of pharmaceutical synthesis, in particular to a preparation method of strontium ranelate, which includes the following steps: reacting 5-amino-4-cyan-3-carbethoxy methyl-thiophene-2-carboxylic acid ethyl ester with a bifunctional alkylating agent in the presence of a catalyst to generate 5-[2-(carbomethoxy)amino]-4-cyan-3-carbethoxy methyl-thiophene-2-carboxylicacid ethyl ester, and then adding strontium chloride aqueous solution after hydrolysis of III in sodium hydroxide aqueous solution for salifying, thus obtaining the strontium ranelate. The preparation method is characterized in that the catalyst is polyethylene glycol, and the bifunctional alkylating agent is methyl chloroacetate or ethyl chloroacetate. In the preparation method, the low-price and environment-friendly catalyst and bifunctional alkylating agent are adopted, so as to be beneficial to industrial production.
Owner:CHINA PHARM UNIV +1
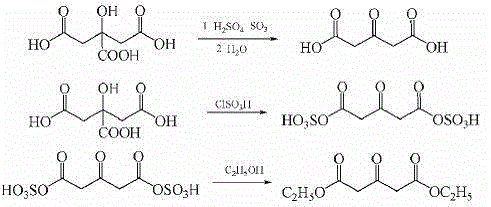
Preparation method of strontium ranelate key intermediate 3-ketoglutaric acid diethyl (dimethyl) ester
InactiveCN103910631ANo emissionsLow costPhysical/chemical process catalystsOrganic compound preparationSodium bicarbonateIce water
The invention discloses a preparation method of strontium ranelate key intermediate 3-ketoglutaric acid diethyl (dimethyl) ester. The method comprises the steps of firstly adding a solvent into a reactor, cooling, putting a solid super acidic catalyst, then putting citric acid monohydrate, slowly heating, performing a thermal reaction, heating until the thermal reaction ends after no obvious bubbles exist, filtering to obtain filtrate, recycling a filter cake as a catalyst, performing solvent recovery on the filtrate, adding into ice water, extracting by adding dichloromethane, washing an extract by using a sodium bicarbonate aqueous solution, water and a saturated saline solution in sequence, and performing reduced pressure distillation to obtain a product, wherein the catalyst is solid super-acid; reaction conditions are as follows: the reaction temperature is between -20 DEG C and 60 DEG C, and the reaction time is 2-12 hours (the sum of the heat preservation time). The process disclosed by the invention does not use any liquid acid catalyst, is mild in reaction, short in steps and simple and convenient in operation, can not cause acid wastewater discharge, and is green and environment-friendly.
Owner:NINGBO LIWAH PHARM CO LTD
Novel method of producing strontium ranelate heptahydrate
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Synthesis method of strontium ranelate and hydrate thereof
ActiveCN101812048AShorten the hydrolysis timeMeet actual needsOrganic chemistryOrganic solventSynthesis methods
The invention provides a synthesis method of strontium ranelate and a hydrate thereof, belonging to the technical field of pharmaceutical synthesis. The synthesis method of the strontium ranelate and the hydrate thereof comprises the steps of reacting ranelate ester (II) with a water solution of an inorganic base under the catalysis of a quaternary ammonium salt in an organic solvent, decoloring, regulating the pH value to a certain range and adding strontium chloride to obtain one of the strontium ranelate and the hydrate thereof. The invention has the advantages that the final product strontium ranelate or the hydrate thereof has high purify and content and can satisfy the requirement of using the strontium ranelate or the hydrate thereof as a pharmaceutical active ingredient.
Owner:浙江东亚药业股份有限公司
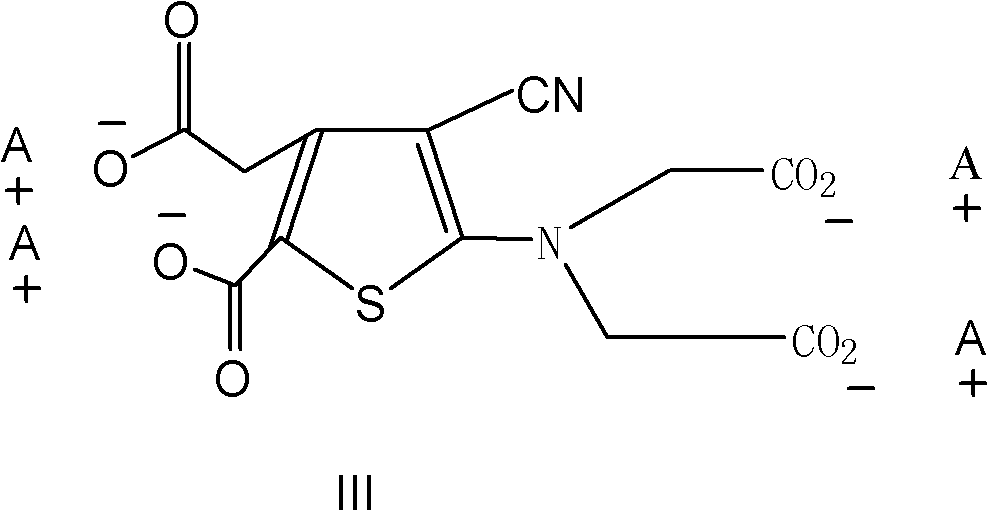
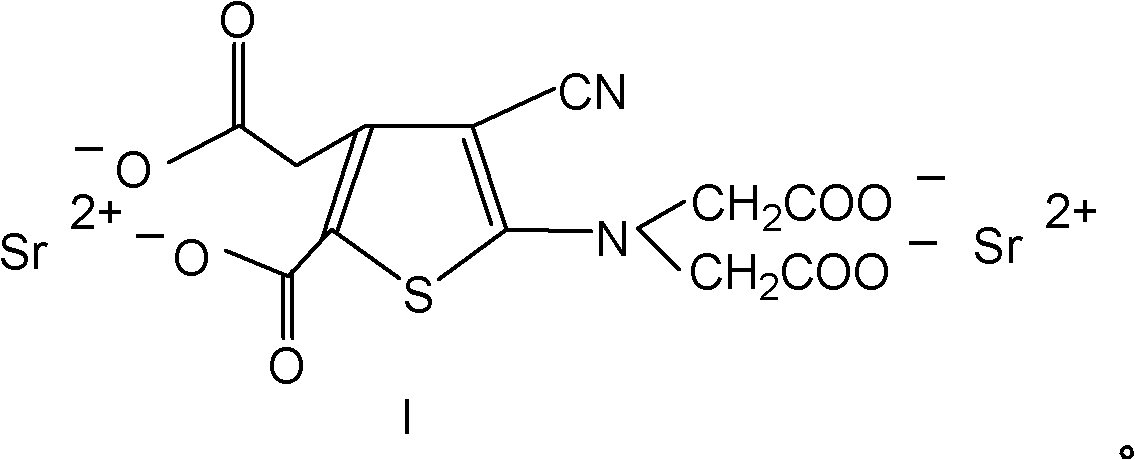
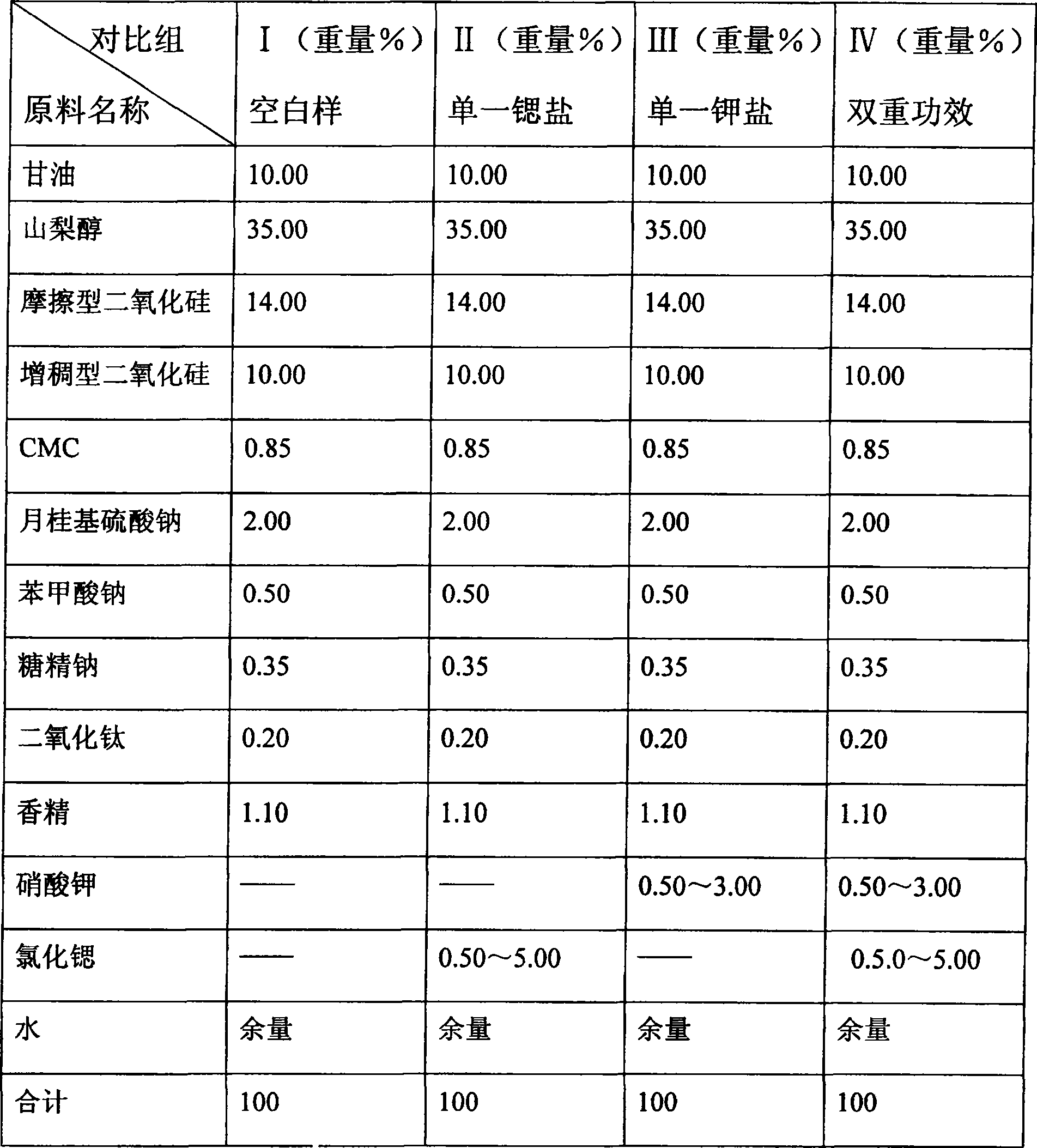
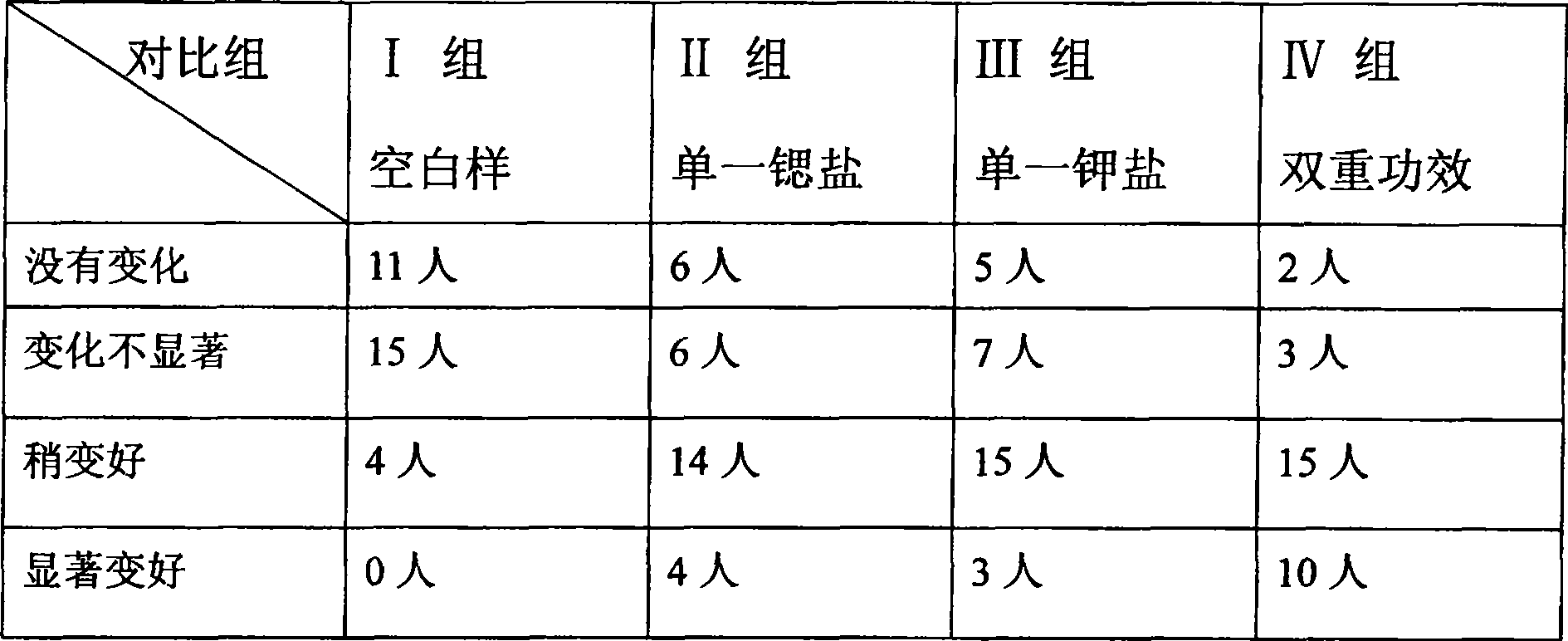
Dual antianaphylaxis tooth paste containing potassium and strontium salt
InactiveCN100502831CImprove stabilityCosmetic preparationsToilet preparationsBarium saltPreservative
The present invention relates to the double antiallergic toothpaste containing potassium salt and strontium salt. In the toothpaste component, it contains humectant, friction agent, binding agent, surfactant, sweetener, spices, preservative, water; it is characterized in that : The toothpaste component contains the whole toothpaste component by weight percentage and is divided into 0.1%~10% strontium salt and 0.1%~5% potassium salt. Tests have shown that the synergistic antiallergic effect of salt and strontium salt is better than that of single potassium salt or strontium salt. Toothpaste containing a single strontium salt or potassium salt cannot achieve the desired effect after three months of use, while toothpaste containing potassium salt and strontium salt can indeed reduce and prevent patients' sensitivity.
Owner:CHONGQING DENCARE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com