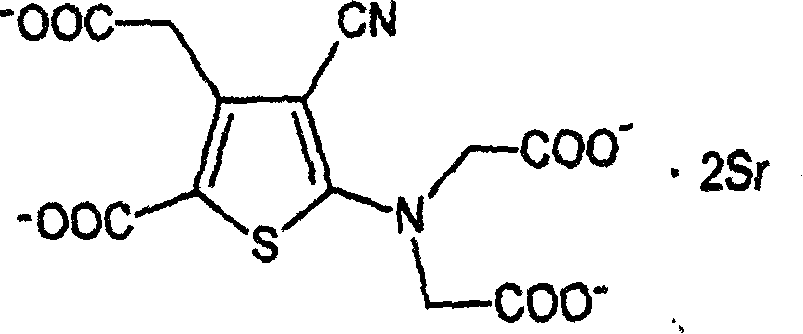
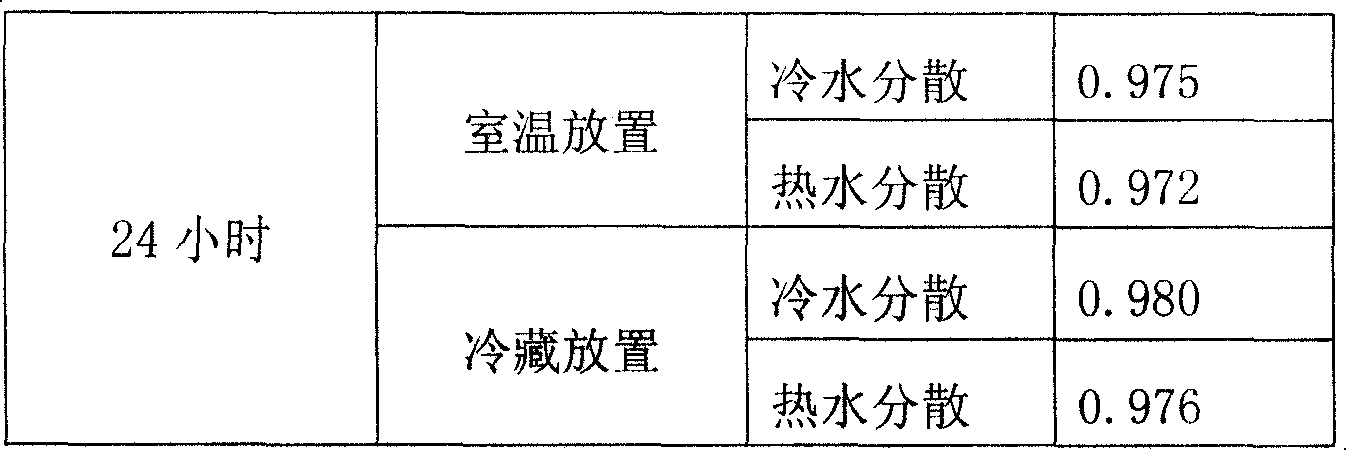
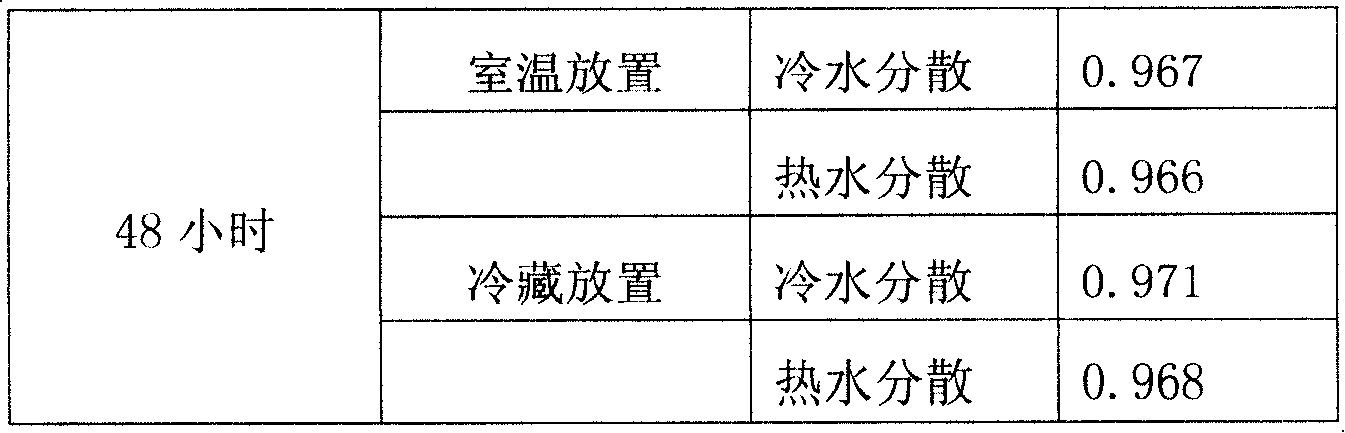
Strontium ranelate dry suspension
A dry suspension, strontium ranelate technology, applied in the direction of organic active ingredients, bone diseases, medical preparations containing active ingredients, etc., can solve the problems that are not suitable for elderly patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prescription composition:
[0020] Strontium ranelate 2000g
[0021] Mannitol 2810g
[0022] Aspartame 10g
[0023] Orange flavor 5g
[0024] lemon yellow 15g
[0025] 15% starch slurry appropriate amount
[0026] Sodium carboxymethyl starch 150g
[0027] Xanthan gum 10g
[0028]
[0029] Make 1000 bags of granules
[0030] Preparation process: Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve, and make starch into 15% starch slurry for later use; mix strontium ranelate and mannitol evenly to obtain mixture I; then mix mixture I with xanthan gum, Sodium carboxymethyl starch is uniformly mixed according to the equal amount incremental method, and an appropriate amount of 15% starch slurry is added to granulate, dried at 50°C, granulated, and classified to obtain granule I, and granule I is mixed with tartrazine, aspartame, and orange essence The mixture II is uniformly obtained, and ...
Embodiment 2
[0046] Strontium ranelate 2000g
[0047] Lactose 1950g
[0048] Sucralose 15g
[0049] Green Apple Flavor 5g
[0050] Fruit green 10g
[0051] Sodium Alginate 20
[0052] 20% maltodextrin pulp appropriate amount
[0053]
[0054] Make 1000 bags of granules
[0055] Preparation process: Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve, and make 20% maltodextrin slurry from maltodextrin for later use; firstly mix strontium ranelate and mannitol evenly to obtain mixture I; then mix mixture I with Sodium alginate is uniformly mixed according to the equal amount incremental method, and an appropriate amount of 20% maltodextrin pulp is added to granulate, dried at 50°C, granulated, and graded to obtain granule I, and granule I is mixed with fruit green, sucralose, and green apple essence The mixture II is uniformly obtained, and the mixture II is divided to obtain a dry suspension.
[0056]...
Embodiment 3
[0070] Prescription composition:
[0071] Strontium ranelate 2000g
[0072] Xylitol 2810g
[0073] Aspartame 10g
[0074] Orange flavor 5g
[0075] lemon yellow 15g
[0076] 15% starch slurry appropriate amount
[0077] Sodium carboxymethyl starch 150g
[0078] Xanthan gum 10g
[0079]
[0080] Make 1000 bags of granules
[0081] Preparation process: Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve, and make starch into 15% starch slurry for later use; mix strontium ranelate and xylitol to obtain mixture I; then mix mixture I and xanthan gum , Sodium carboxymethyl starch is mixed uniformly by equal amount incremental addition method, and an appropriate amount of 15% starch slurry is added to granulate, dried at 50°C, granulated, and graded to obtain granule I, and granule I is mixed with tartrazine, aspartame, and orange essence Mix evenly to obtain the mixture II, and divide the mixture I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com