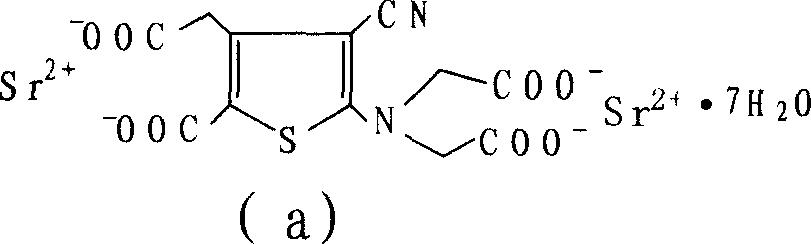
Novel method of producing strontium ranelate heptahydrate
A technology of heptahydrate and strontium ranelate, applied in the field of new preparation of strontium ranelate heptahydrate, can solve the problems of unstable water content of hydrate, troublesome practical operation, difficult purification operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of strontium ranelate heptahydrate
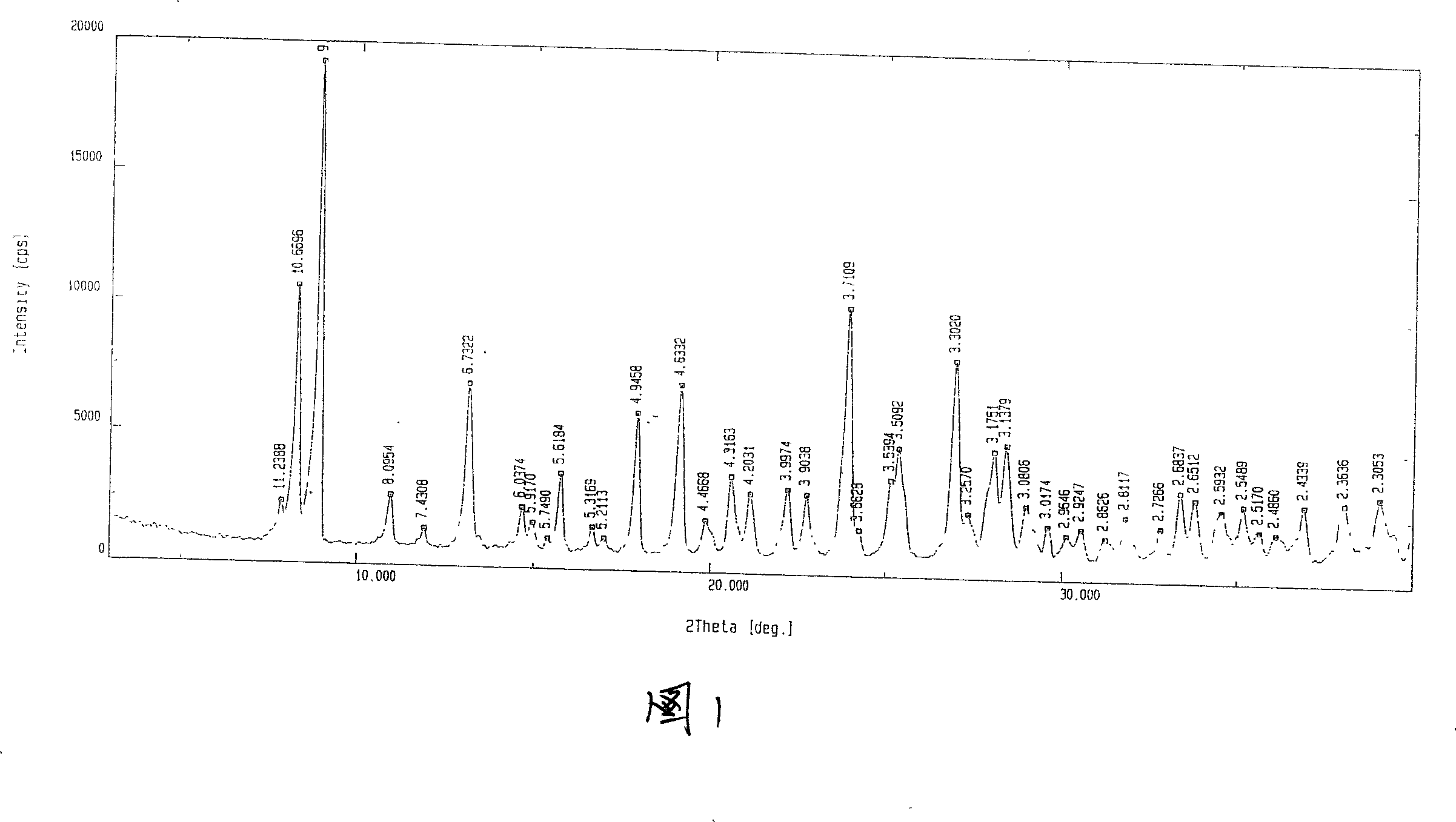
[0056] Weigh 400g of 2-[N,N-di(carboxymethyl)amino]-3-cyano-4-carboxymethylthiophene-5-carboxylate tetraethyl ester into the reaction flask, add 3.6L1N sodium hydroxide solution and 3.6L ethanol, heated to reflux, reacted for 4h, kept the pH value above 10 during the reaction, lowered the temperature, filtered, evaporated the filtrate to remove ethanol under reduced pressure, quantitatively added 720ml ethanol to the aqueous solution, and then added 2 times the molar amount of strontium chloride water solution, a solid precipitated. Filter, wash the filter cake layer with water 3 times, drain it, dry it in the air, add 4L of water to reflux, heat filter, wash it 3 times with boiling water, drain it, place it at room temperature, and keep the weight constant to obtain 440g. Loss on drying 19.82%, related substances 0.17%. The XRD pattern of strontium ranelate heptahydrate crystal is shown in the accompanyin...
Embodiment 2
[0057] Embodiment 2: Preparation of strontium ranelate heptahydrate
[0058]Weigh 2-[N,N-di(carboxymethyl)amino]-3-cyano-4-carboxymethylthiophene-5-carboxylic acid tetraethyl ester, add 500g into the reaction flask, add 4.5L1N sodium hydroxide solution and 4.5L ethanol, heated to reflux, reacted for 8 hours, kept the pH value at 13, cooled down, filtered, the filtrate was evaporated to remove ethanol under reduced pressure, 2250ml ethanol was added to the aqueous solution, and then a solution of 2.5 times the molar amount of strontium chloride water was added to precipitate solid. Filter, wash the filter cake layer with water 3 times, drain it, dry it in the air, add 5L of water to reflux, heat filter, wash it 3 times with boiling water, drain it, place it at room temperature, and obtain 560g at constant weight. Loss on drying 19.74%, related substances 0.08%. The XRD pattern of strontium ranelate heptahydrate crystal is shown in the accompanying drawing 1 of the description...
Embodiment 3
[0059] Embodiment 3 pharmaceutical composition
[0060] Prepare 1000 bags of granule prescriptions, each containing 1g of active ingredient
[0061] The crude drug 1246g of embodiment 1
[0062] Lactose 1800g
[0063] Microcrystalline Cellulose 1500g
[0064] Xylitol 100g
[0065] Sorbitol 100g
[0066] Methylcellulose 200g
[0067] Flavor 100g
[0068] Preparation Process:
[0069] Pass the main ingredient and auxiliary materials through a 200-mesh sieve respectively. Fully mix the excipients first, then weigh the prescribed amount of excipients and mix them fully with the main drug. Use 10% pvp water to make soft material, granulate with 20 mesh sieve, dry at 50°C, granulate with 20 mesh sieve, sieve out fine powder, and pack.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com