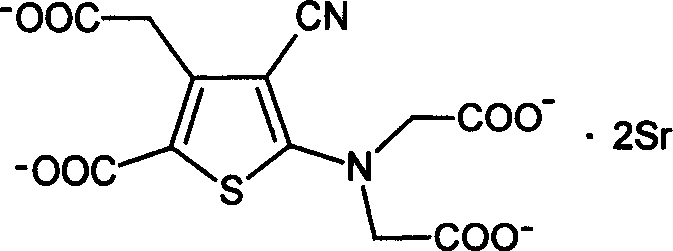
Medicinal composition containing strontium fuminate and vitamin D
A technology of strontium ranelate and vitamins, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problems of patients forgetting, complicated taking methods, and reduced compliance, and achieve convenient drug use and promote calcium Absorption, promoting the effect of bone calcification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1——compound strontium ranelate vitamin D chewable tablet
[0026] Strontium ranelate 1000g
[0027] Vitamin D3 200,000 IU
[0028] Mannitol 500g
[0029] Aspartame 30g
[0030] 10% starch slurry appropriate amount
[0031] Sweet Orange Flavor 50g
[0032] Micronized silica gel 10g
[0033] Made into 1000 pieces
[0034] The above prescription amounts of strontium ranelate, vitamin D3, mannitol and aspartame are mixed evenly in equal amounts, and 10% starch slurry is made into a soft material, which is then granulated, dried, granulated, and then mixed with essence and micro-powdered silica gel Evenly, and then pressed into pieces.
[0035] Application: the pharmaceutical composition obtained above is applied to treat postmenopausal osteoporosis and reduce the risk of vertebral and hip bone fractures. Chew orally, once a day, 2 tablets at a time, preferably two hours after eating and before going to bed.
Embodiment 2
[0036] Embodiment 2——compound strontium ranelate vitamin D granules
[0037] Strontium ranelate 2000g
[0038]Vitamin D3 800,000 IU
[0039] Mannitol 1200g
[0040] Dextrin 400
[0041] Aspartame 35g
[0042] Make 1000 bags
[0043] After mixing the raw and auxiliary materials in the above prescription amount evenly, granulate with water or 20% ethanol solution, dry the granules, granulate, then sieve into granules of appropriate size, and pack them into bags.
[0044] Application: the pharmaceutical composition obtained above is applied to treat postmenopausal osteoporosis and reduce the risk of vertebral and hip bone fractures. Take orally, once a day, 1 bag at a time, preferably two hours after eating and before going to bed. Stir each sachet with water before use, and then take it.
Embodiment 3
[0045] Example 3—Composite packaging of strontium ranelate single-component preparation and vitamin D single-component preparation
[0046] (1) Preparation of strontium ranelate chewable tablets
[0047] Strontium ranelate 1000g
[0048] Lactose 500g
[0049] Glycyrrhizin 30g
[0050] Sodium cyclamate 15
[0051] 5% polyvinylpyrrolidone 50% ethanol solution appropriate amount
[0052] Apple essence 50g
[0054] Made into 1000 pieces
[0055] The above prescription amount strontium ranelate, lactose, glycyrrhizin and sodium cyclamate are mixed evenly, 5% polyvinylpyrrolidone 50% ethanol solution is made into a soft material, and then granulated, dried, granulated, and then mixed with apple essence and The talcum powder is mixed evenly, and then compressed into tablets.
[0056] (2) Preparation of strontium ranelate particles
[0057] Strontium ranelate 2000g
[0058] Mannitol 1200g
[0059] Dextrin 400
[0060] Aspartame 35g
[0061] Make 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com