Novel method for preparing substituted thenoic acid ester and uses thereof
A technology of thiophene formate and chloroacetic acid, applied in directions such as organic chemistry, can solve the problems of increased manufacturing cost, increased risk of environmental damage, and high cost of bromoacetate and phase transfer catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
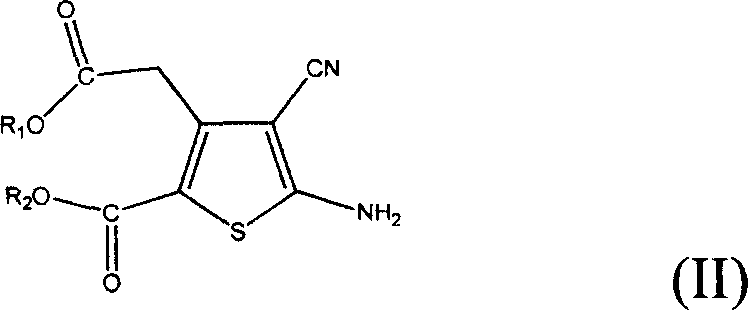
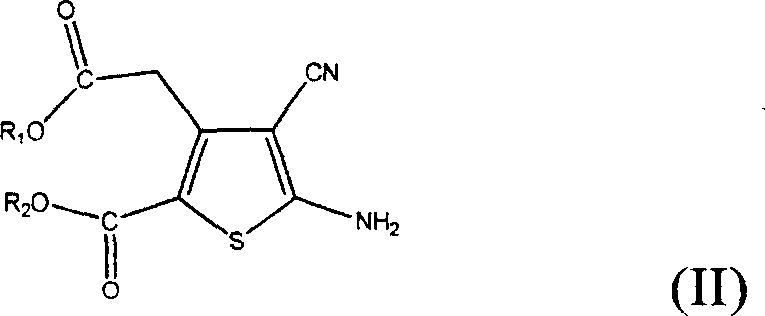
[0026] Example 1: 5-Amino-3-(2-ethoxy-2-oxoethyl)-4-cyano-2-thiophenecarboxylic acid ethyl ester
[0027] 700 milliliters of ethanol, 500 grams of diethyl 3-oxoglutarate and 200 grams of malononitrile were respectively added into the reaction flask, stirred vigorously, 250 grams of morpholine were added, and stirred for 1 hour. Add 100 grams of sulfur powder and heat to reflux. The reaction was kept at reflux for 3 hours, cooled to room temperature, poured into 1500 ml of water, and a large amount of solids were precipitated. Filter, wash with 200 ml of water, and air-dry at 50°C until constant weight to obtain 520 g of light yellow solid, yield: 75%.
Embodiment 2
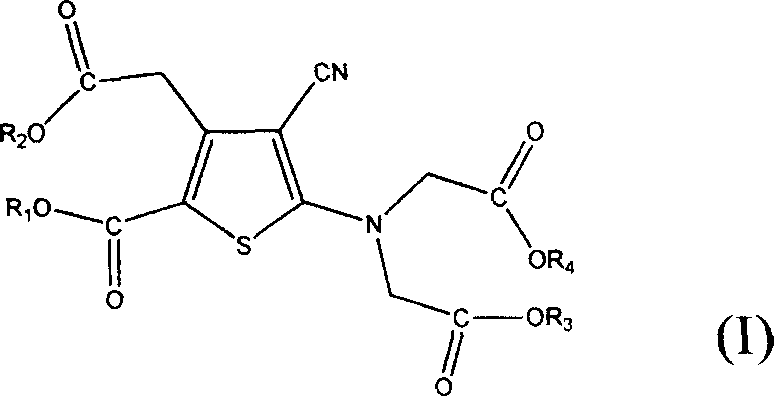
[0028] Example 2: 5-[Bis(2-ethoxy-2-oxoethyl)amino-3-(2-ethoxy-2-oxoethyl)-4-cyano-2-thiophenecarboxylic acid ethyl ester
[0029] Add 500 grams of ethyl 5-amino-3-(2-ethoxy-2-oxoethyl)-4-cyano-2-thiophenecarboxylate prepared according to Example 1 to 3000 ml of acetone, add 20 grams of trimethylbenzyl ammonium chloride, 600 grams of ethyl chloroacetate, and 500 grams of anhydrous potassium carbonate were stirred and heated to reflux. Keep the reflux reaction for 12 hours, cool to room temperature, filter, and concentrate the filtrate to dryness. Add 1000 ml of methanol, heat to reflux for 1 hour, stir in an ice-water bath for 5 hours, filter, and blow dry at 50° C. to constant weight to obtain 640 grams of white solid, yield: 80%.
Embodiment 3
[0030] Example 3: Strontium ranelate
[0031] 5-[bis(2-ethoxy-2-oxoethyl)amino-3-(2-ethoxy-2-oxoethyl)-4-cyano-2 prepared according to Example 2 - Add 300 grams of ethyl thiophenecarboxylate to 3000 milliliters of water, add 350 grams of strontium hydroxide, stir, and heat to reflux. Keep the reflux reaction for 6 hours, filter, and rinse with 300 ml of hot water. Vacuum-dried at 80°C to constant weight to obtain 315 g of white solid, yield: 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com