Preparation method of strontium ranelate
A technology of strontium ranelate and ethyl carboxylate, applied in the field of drug synthesis, can solve the problems of insoluble, difficult to purify, disparity and the like of strontium ranelate, and achieves the effects of low price and little environmental harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
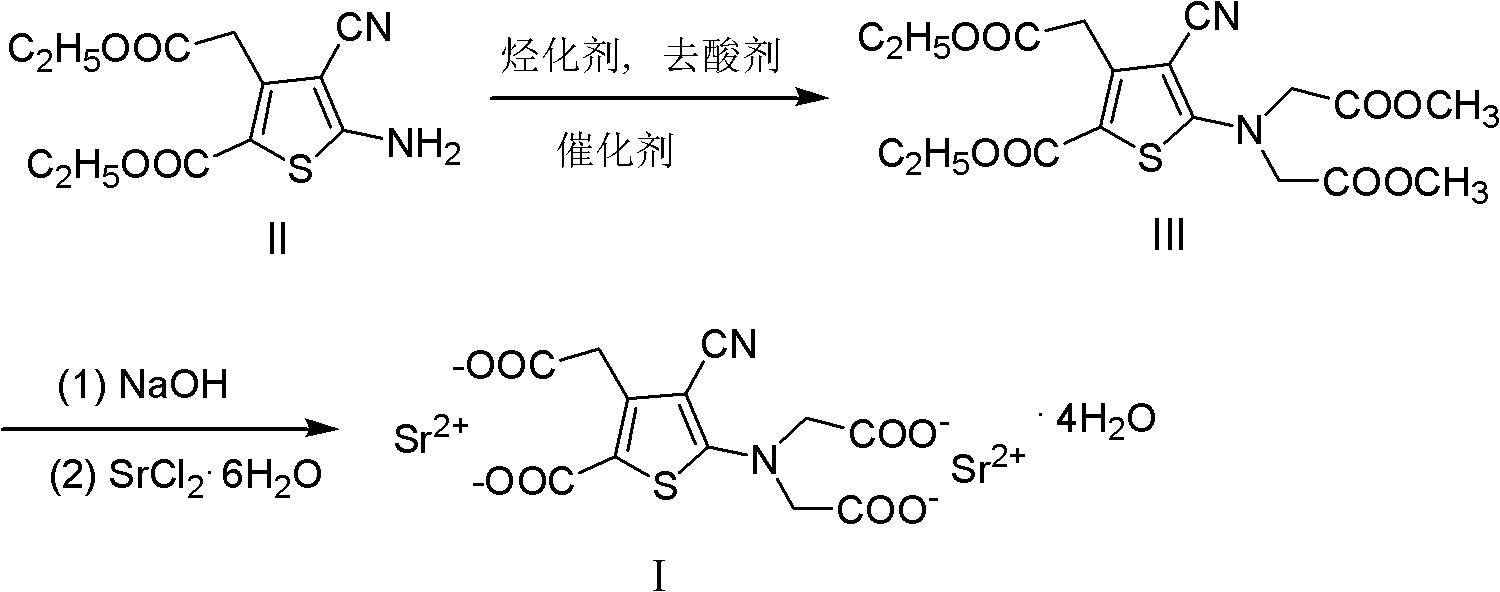
[0017] 5-Amino-4-cyano-3-ethoxycarbonylmethyl-thiophene-2-carboxylic acid ethyl ester (II)
[0018] In a 1000ml four-necked bottle, add 166.8g (0.825mol) of diethyl 3-oxoglutarate, 56.7g (0.858mol) of malononitrile and 200ml of ethanol, stir mechanically, and add dropwise morpholine 71.9g under ice water cooling g (0.825mol), the control temperature is not higher than 20°C. After dripping, stir for 10 minutes, add sulfur 26.5g (0.825mol), and reflux for 2.5 hours. Stop reflux, add dropwise 300ml of water, gradually precipitate, continue to stir for 20 minutes after dropping, cool, filter with suction, filter cake is washed with about 70ml of ice-cold 50% (v / v) ethanol, and dry to obtain crude product (189.8g, 81.5% ), recrystallized twice with 50% (v / v) ethanol to obtain 148.7 g (63.8%) of light khaki crystal II, mp.137-138°C.
Embodiment 2
[0020] 5-[Di-(methoxycarbonylmethyl)amino]-4-cyano-3-ethoxycarbonylmethyl-thiophene-2-carboxylic acid ethyl ester (III)
[0021] In a 2000ml reaction flask, add II 127g (0.450mol), polyethylene glycol 600 8.1g (0.0135mol), anhydrous potassium carbonate 136.8g (0.99mol), acetone 760ml and methyl chloroacetate 107.4g (0.99mol) , stirred and refluxed for 10 hours, cooled to room temperature, and filtered off inorganic salts. The filtrate was concentrated under reduced pressure with acetone until it was exhausted, and 300ml of methanol was added to dissolve it and left to stand overnight, suction filtered, and dried at 60°C to obtain 169.3g (88.2%) of crystals, which were recrystallized with 70% (v / v) ethanol to obtain 156.6g of white crystals ( 81.6%), mp. 106-107°C.
Embodiment 3
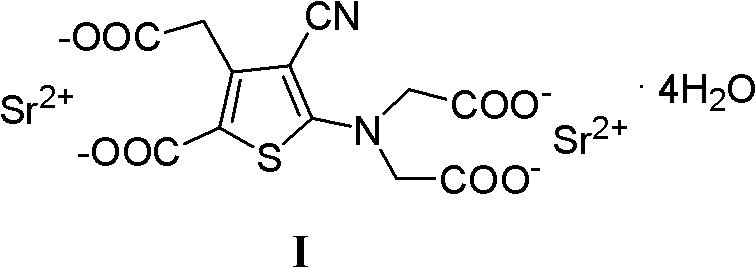
[0023] Strontium(I) ranelate
[0024] In a 2000ml reaction flask, add 128g (0.3mol) of III and 600ml of water, stir mechanically, add a solution composed of 50.4g (1.26mol) of sodium hydroxide and 600ml of water, reflux for 8 hours, adjust the pH to 8.5~ with concentrated hydrochloric acid 9.5, after cooling to room temperature, extract twice with ethyl acetate, 500ml each time, add activated carbon to the water layer for decolorization, filter, add SrCl to the filtrate 2 ·6H 2 0 and 330ml of water, stirred and refluxed for 1 hour, suction filtered while it was hot, and the resulting solid was placed in 600ml of 80% ethanol, heated and refluxed for 30 minutes, filtered while it was hot, and the solid was washed twice with water at about 80°C, 300ml each time, Dry at 55°C to obtain 155.2 g of white solid powder with a yield of 88.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com