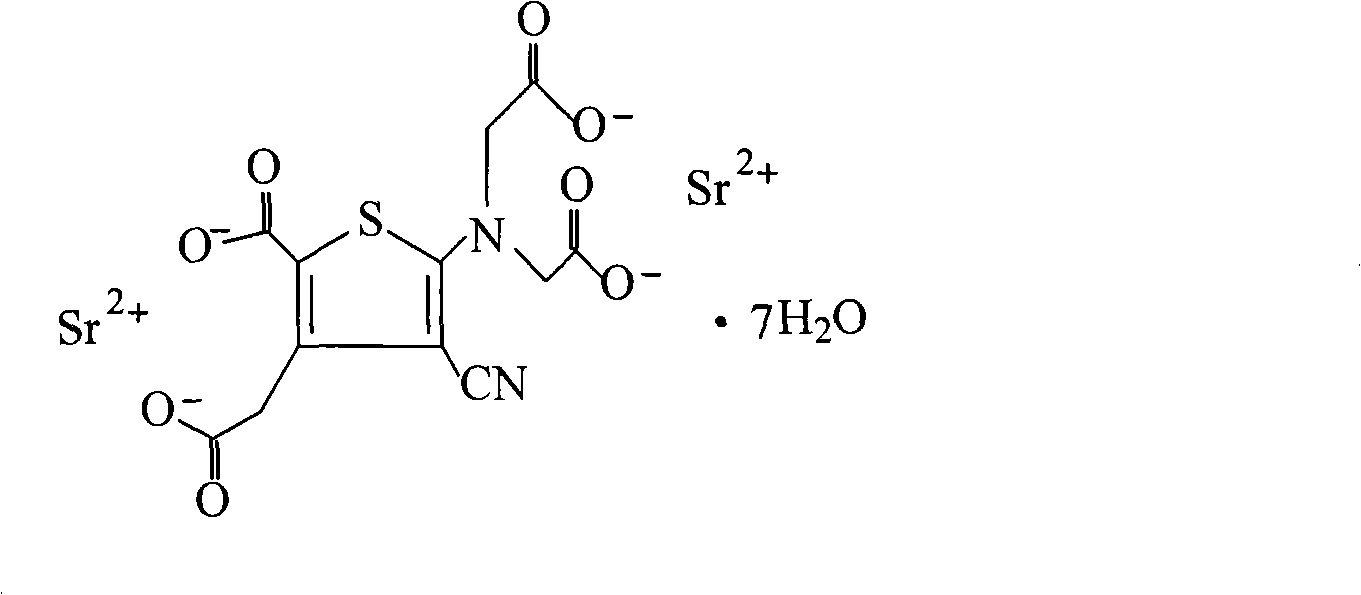
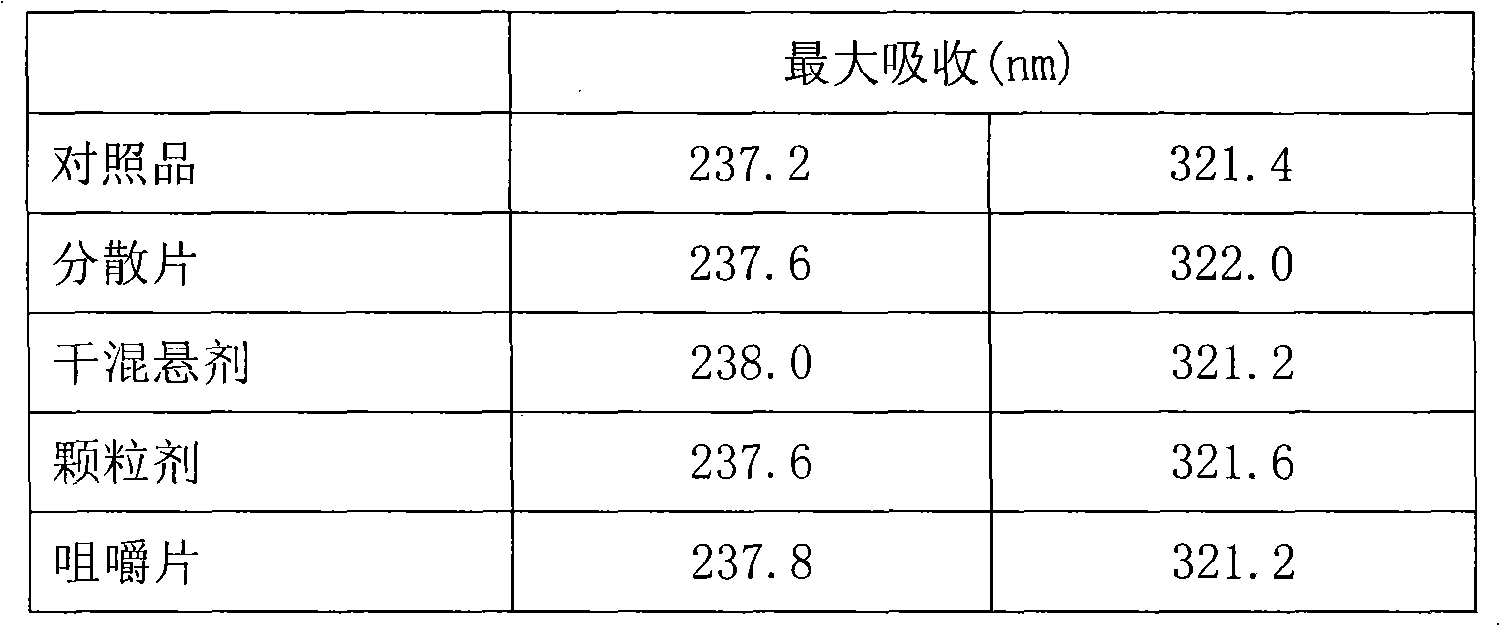
Pharmaceutical combination with stable strontium ranelate and its preparations
A technology of strontium ranelate and a composition, which is applied in the field of strontium ranelate-stabilized pharmaceutical compositions and preparations thereof, can solve problems such as increase in related substances, appearance and color change, etc., and achieves simplified operation and is suitable for large-scale industrial production. , the effect of shortening the synthesis route of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037]
[0038] Preparation Process:
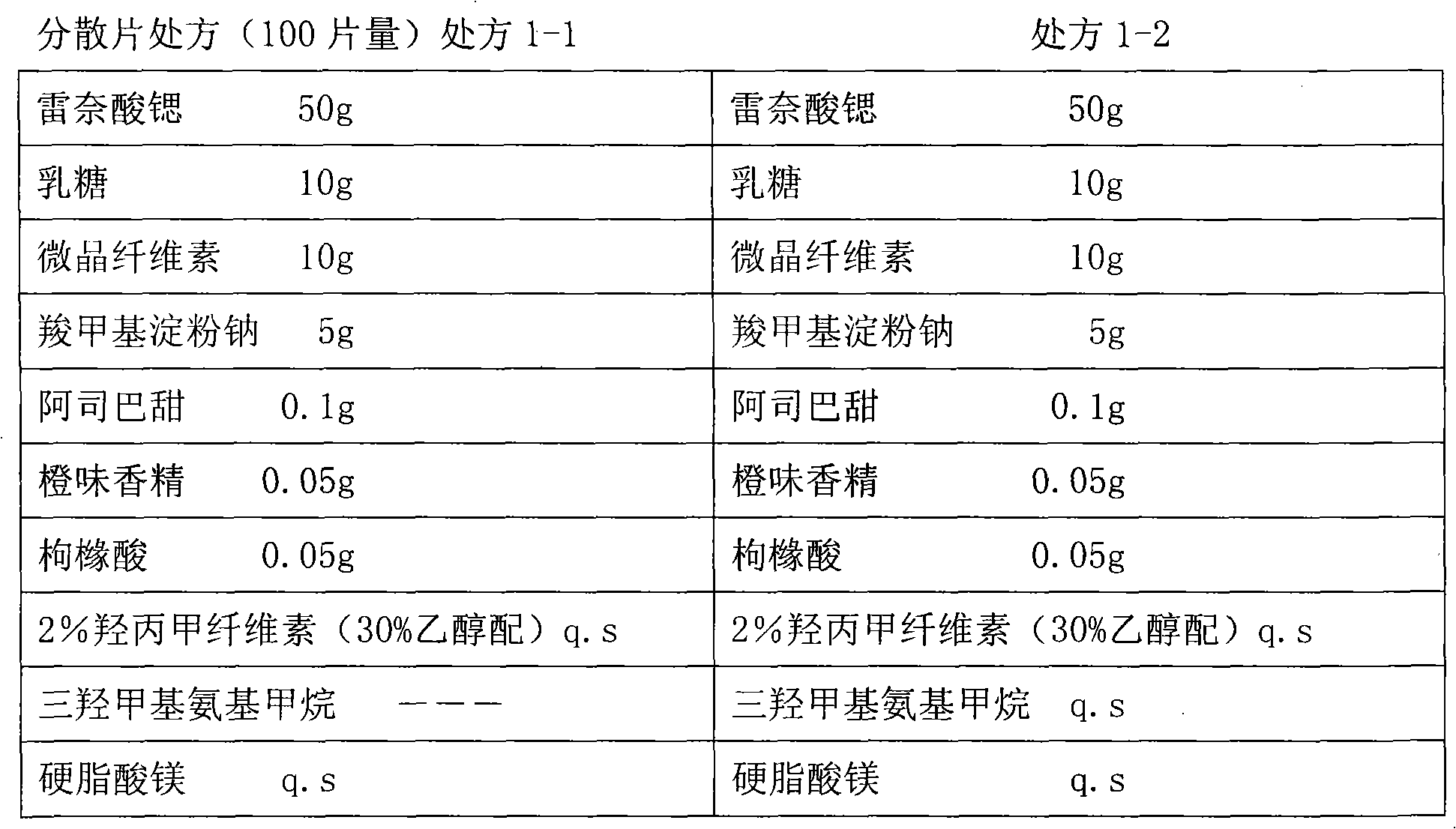
[0039] The raw and auxiliary materials are respectively passed through a 100-mesh sieve for subsequent use. Mix the prescription amount of excipients thoroughly first. Add the raw material medicine to the excipients, so that the medicine and the excipients are fully mixed. Add an appropriate amount of trishydroxymethyl aminomethane to the binder containing 2% hypromellose (30% ethanol), add the binder to the mixture to make a soft material, pass through a 20-mesh sieve and granulate , dried in a ventilated oven at 55°C for 2 hours, passed through a 18-mesh sieve to granulate the dry granules, determined the content of intermediates, and pressed into tablets.
example 2
[0041] Chewable Tablet Prescription (100 Tablets)
[0042] Strontium ranelate 100g
[0043] Mannitol 60g
[0044] Microcrystalline Cellulose 10g
[0045] Xylitol 0.1g
[0046] 10% povidone (water) q.s
[0047] Sodium Lauryl Sulfate 0.15g
[0049] The preparation process is the same as in Example 1.
example 3
[0051] Orally disintegrating tablet prescription (100 tablets)
[0052] Strontium Ranelate 25g
[0053] Mannitol 40g
[0054] Microcrystalline Cellulose 10g
[0055] Crospovidone 12g
[0056] Aspartame 0.1g
[0057] Strawberry essence 0.05g
[0058] 2% hypromellose (30% ethanol) q.s
[0059] Magnesium Lauryl Sulfate 1.8g
[0061] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com