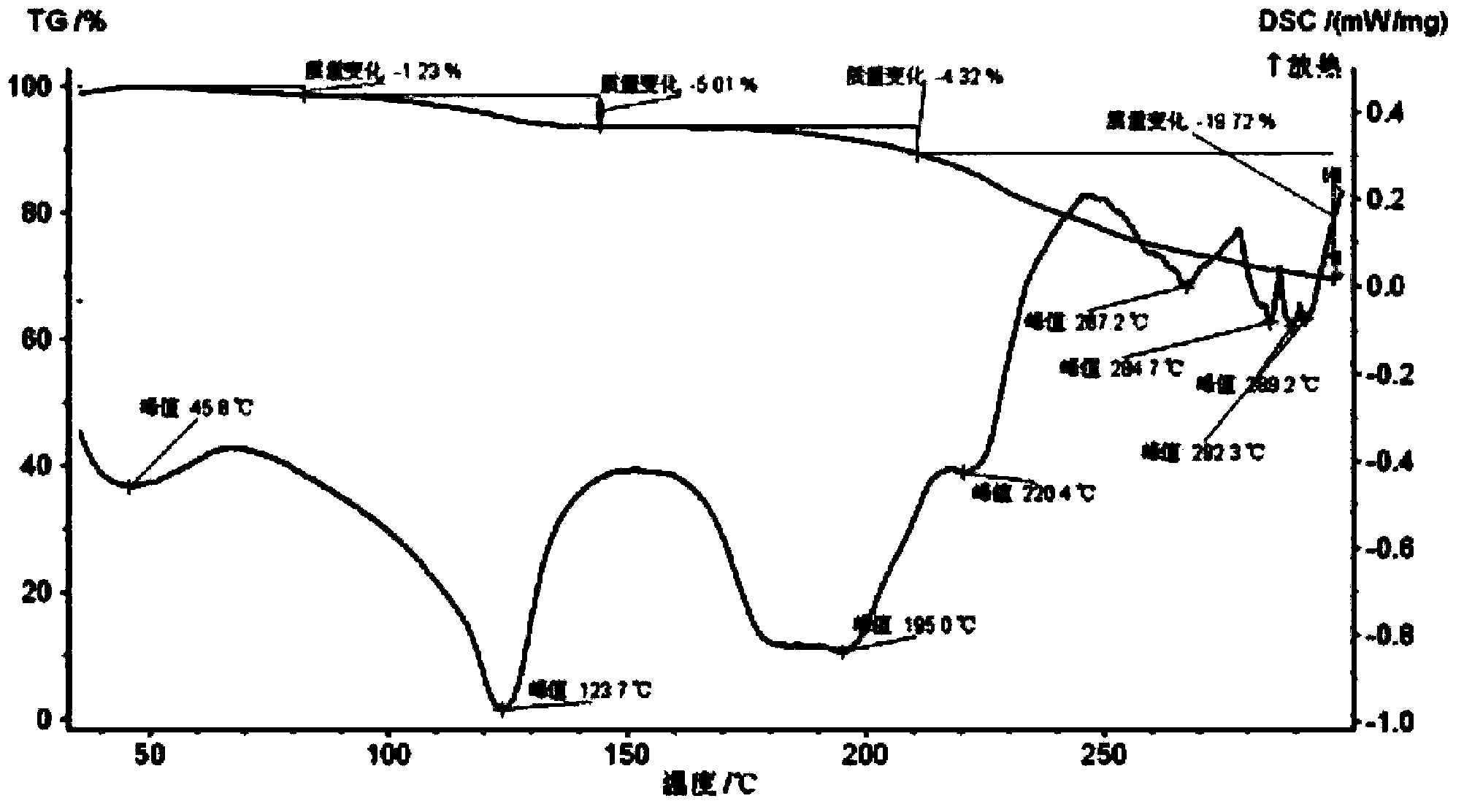
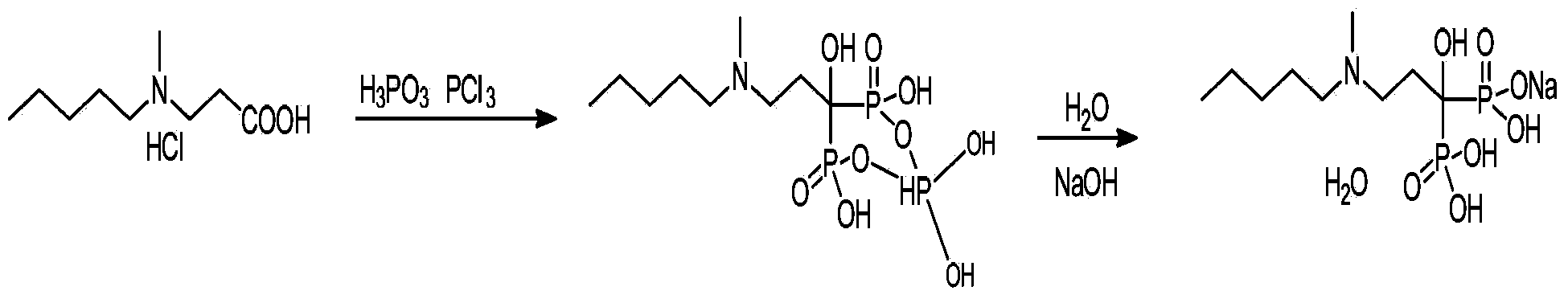
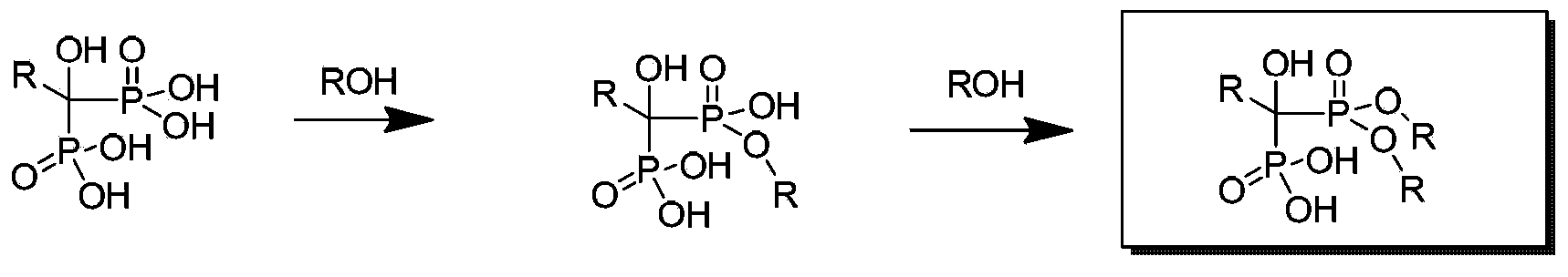
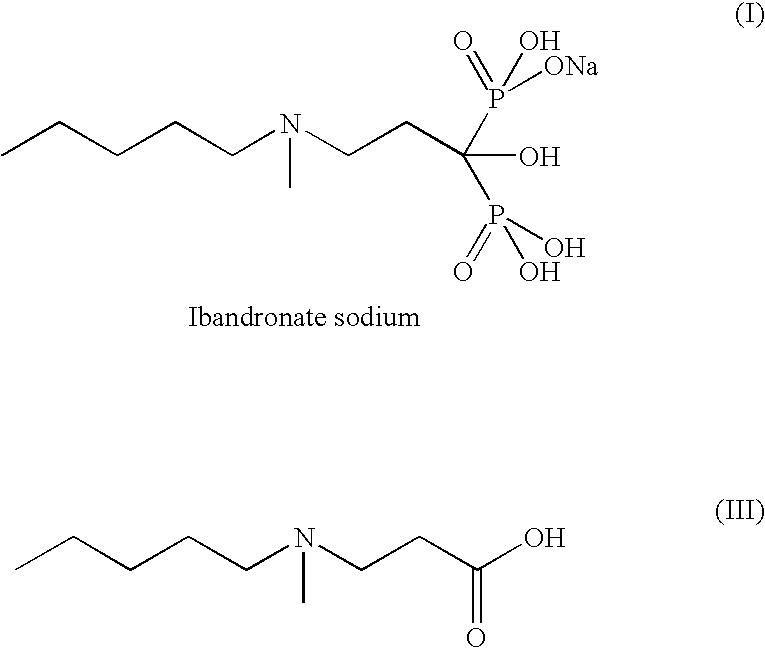
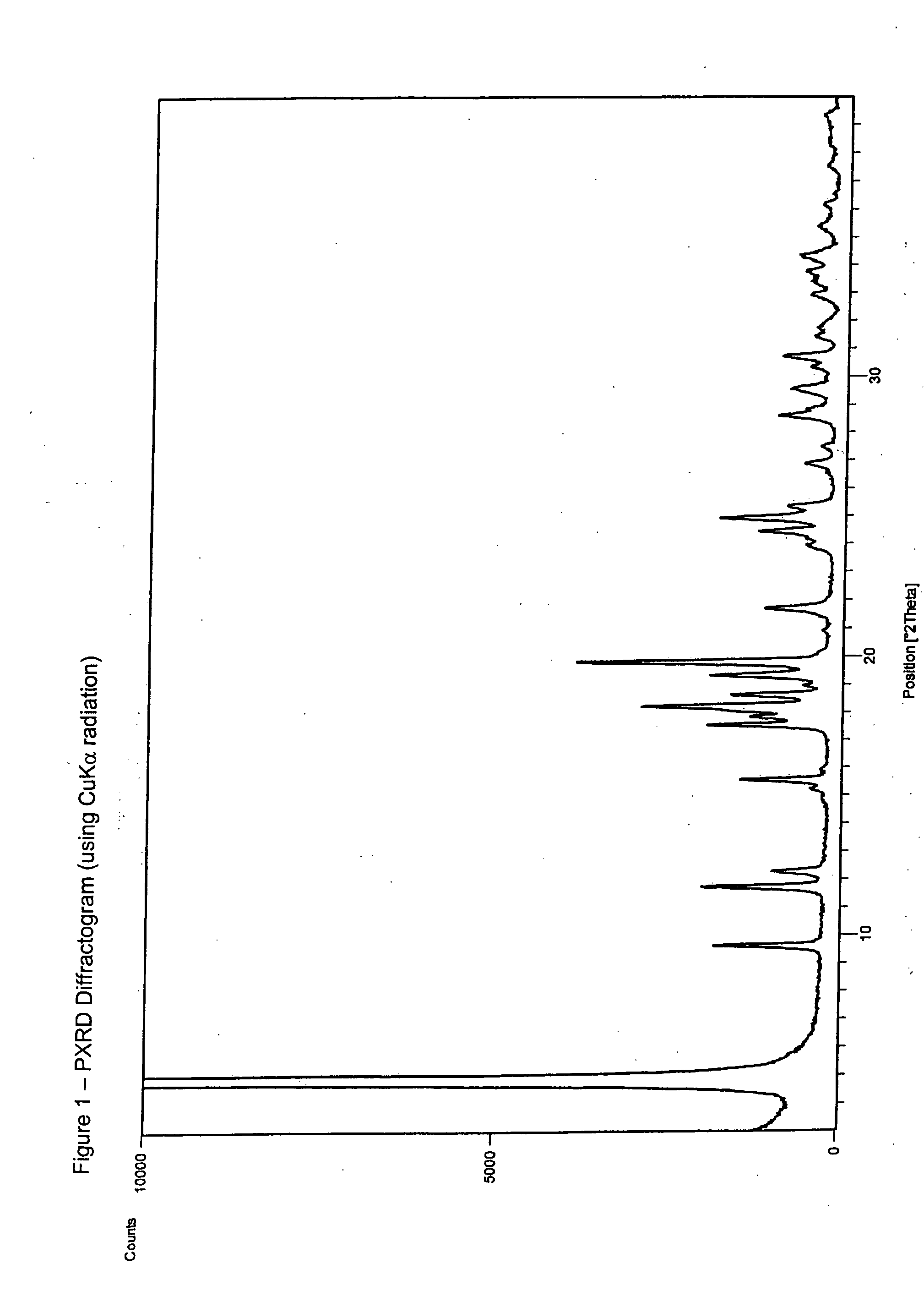
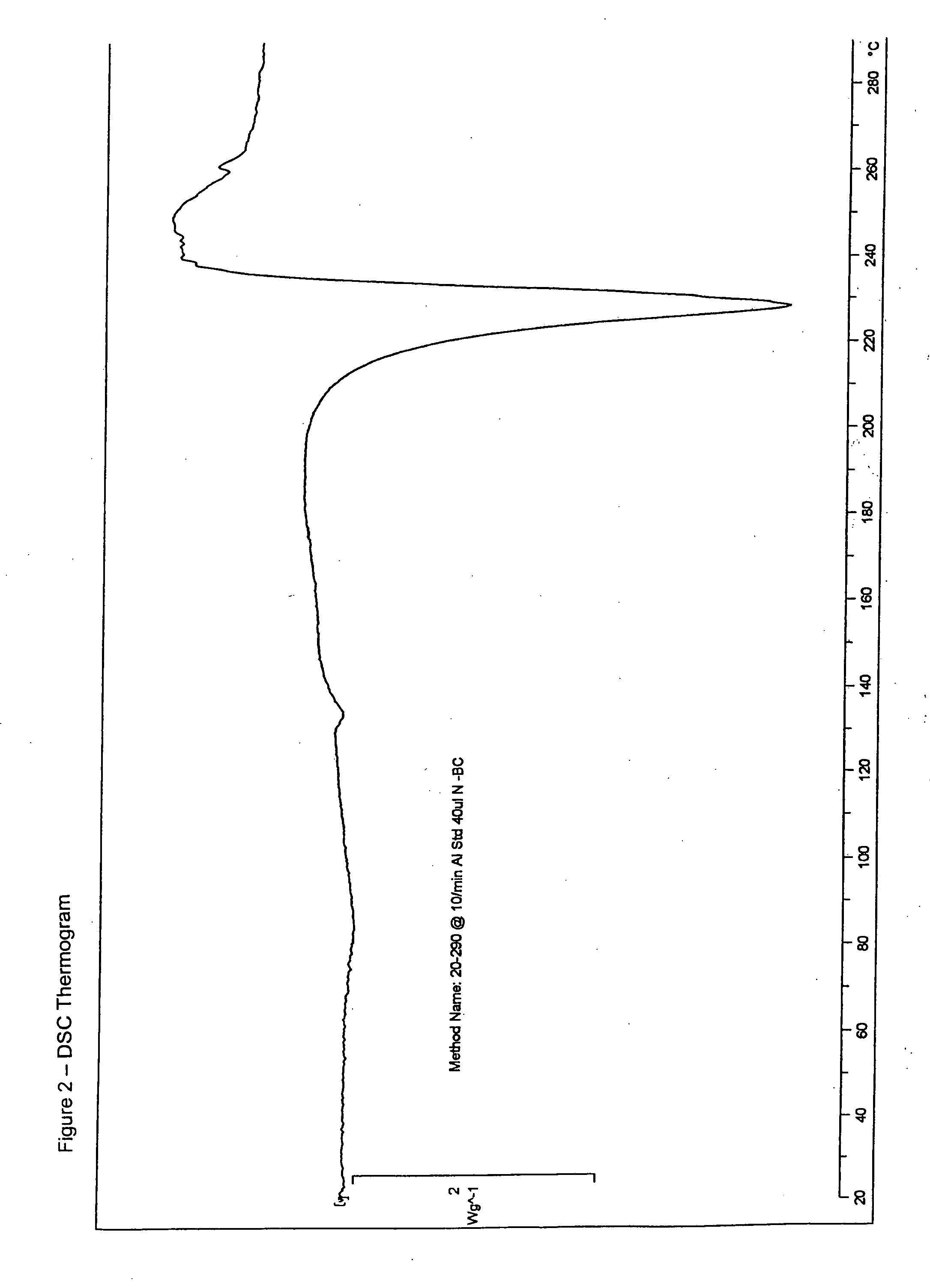
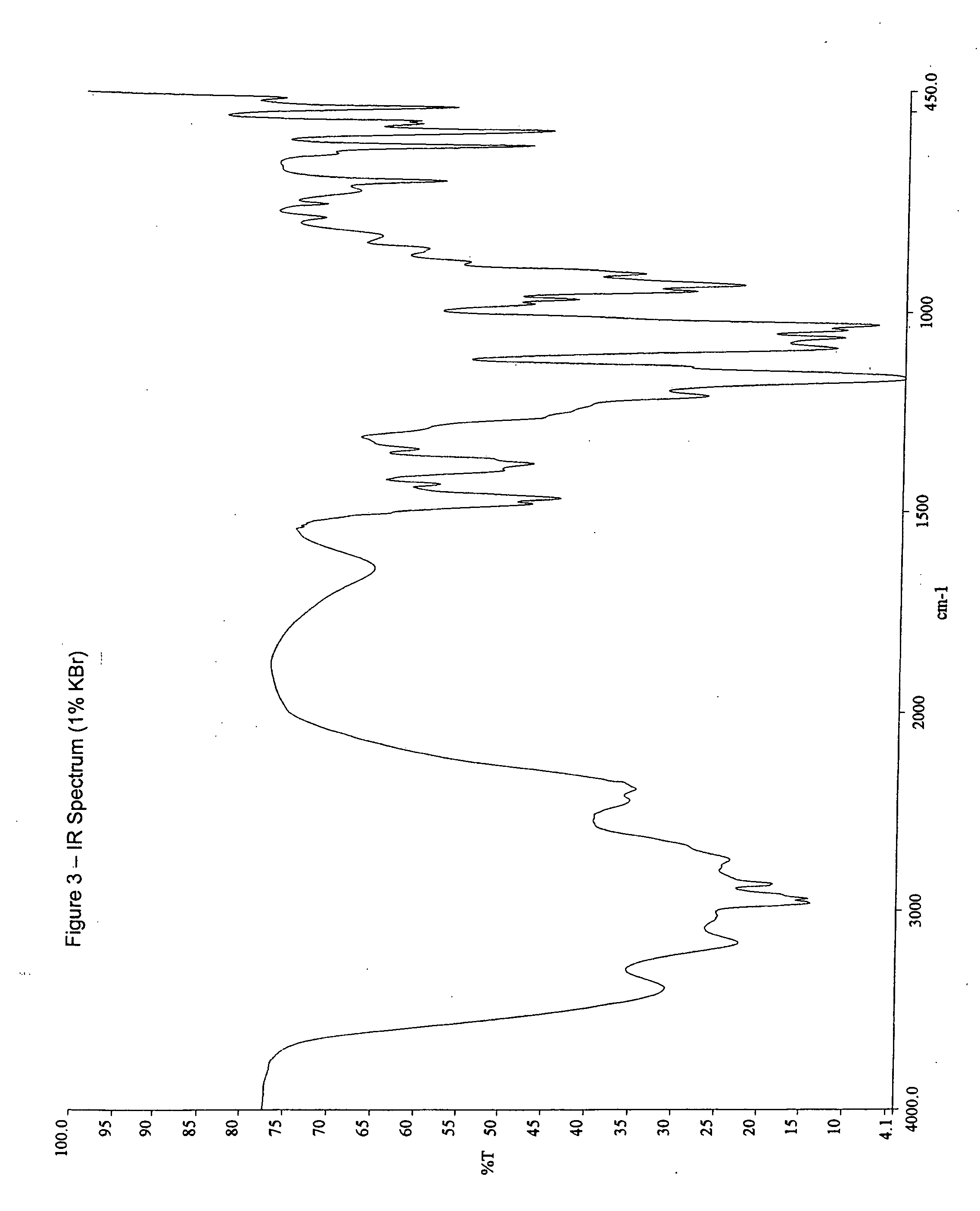
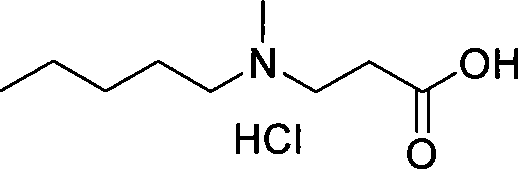
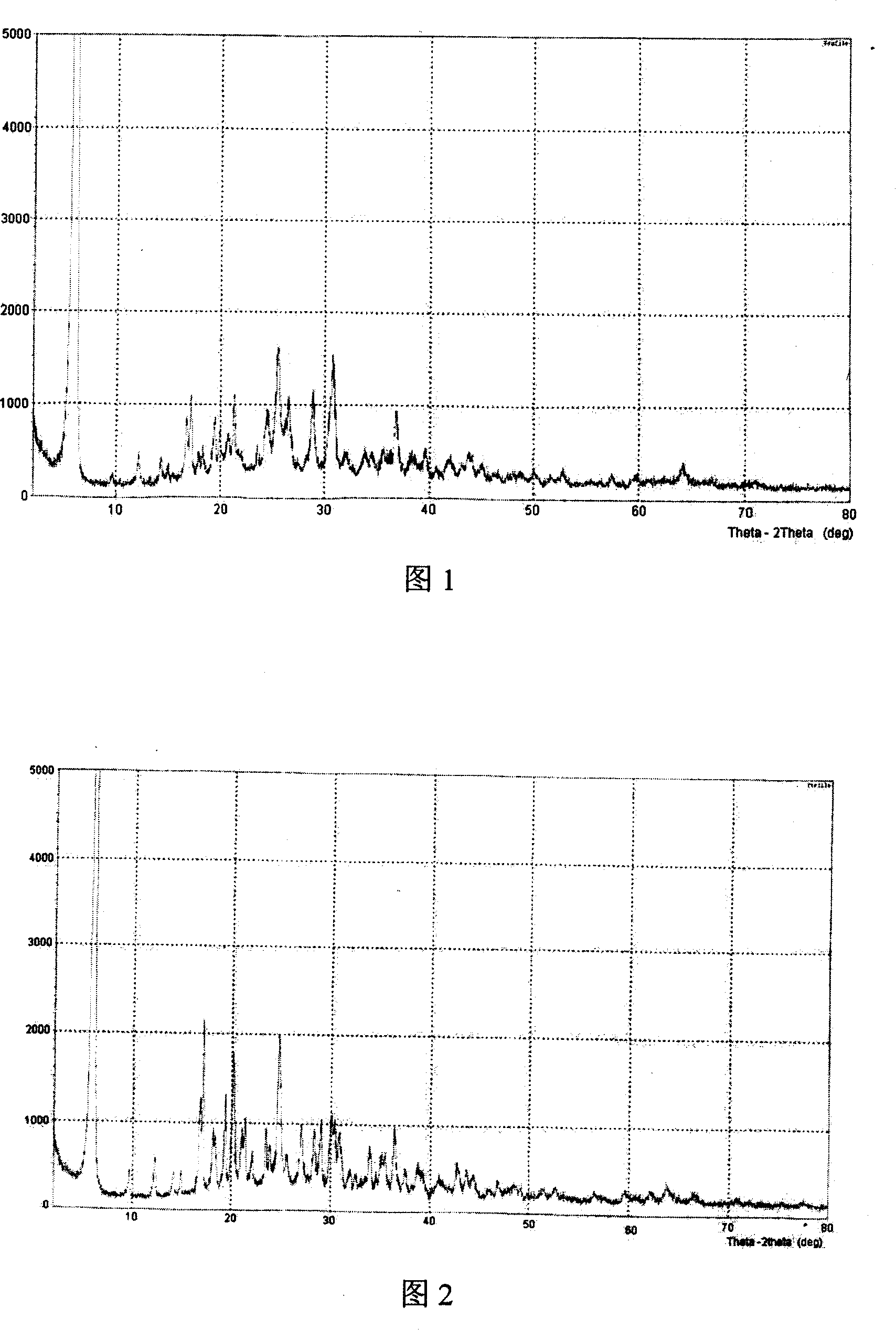
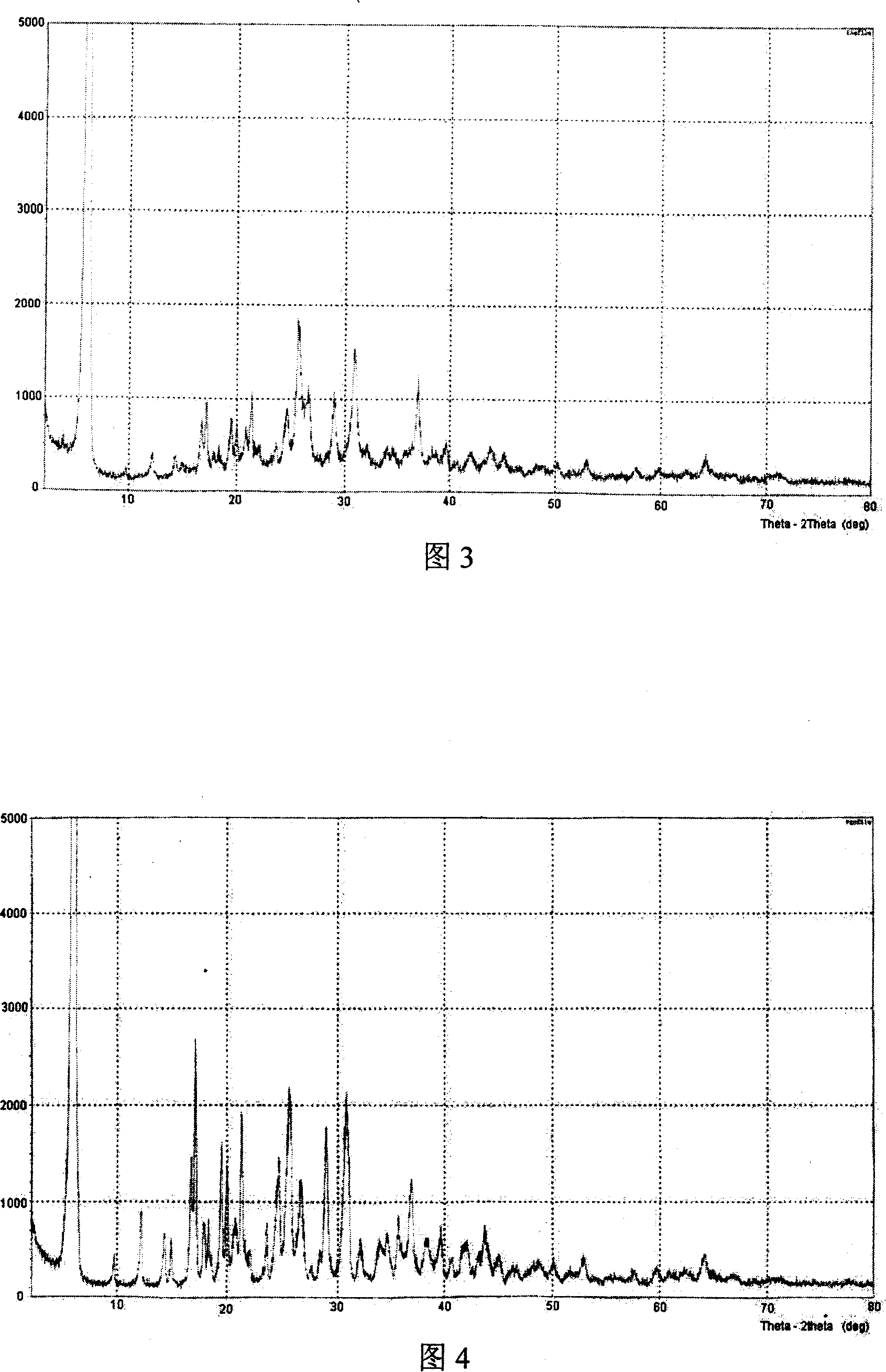
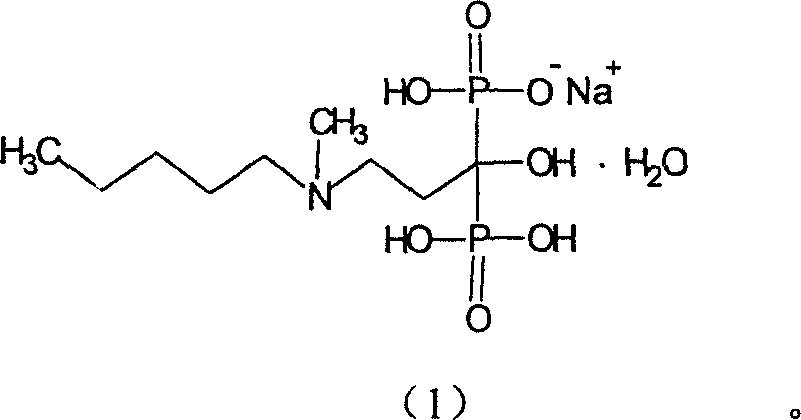
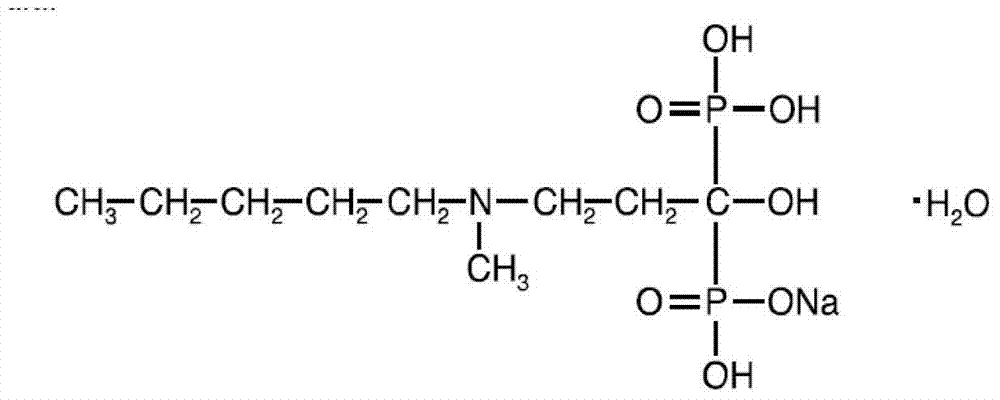
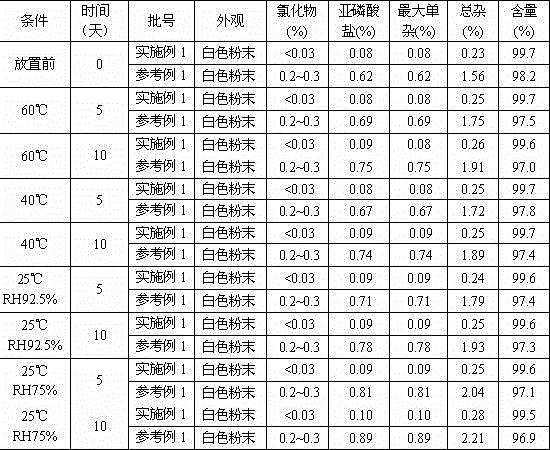
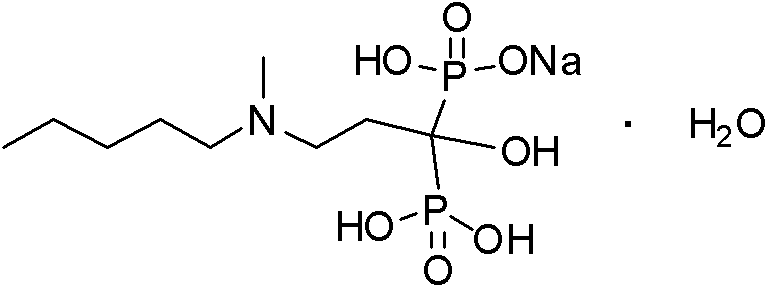
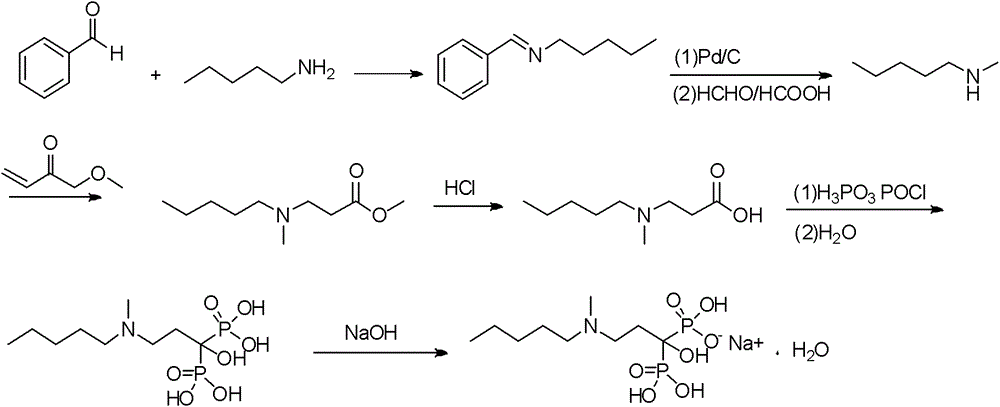
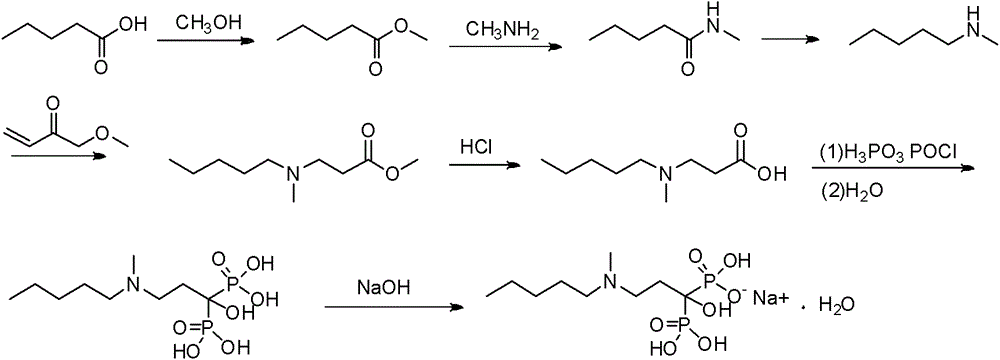
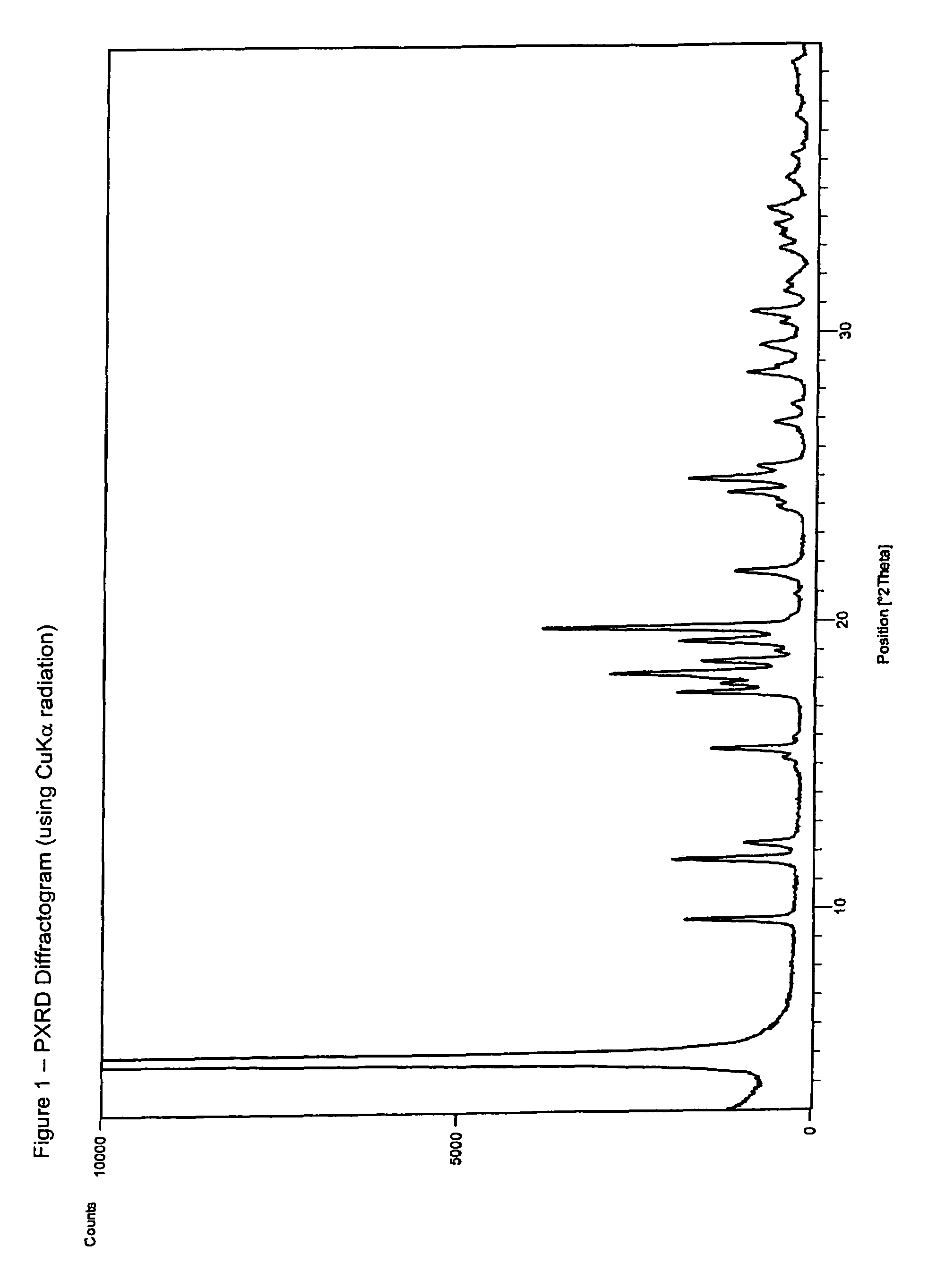
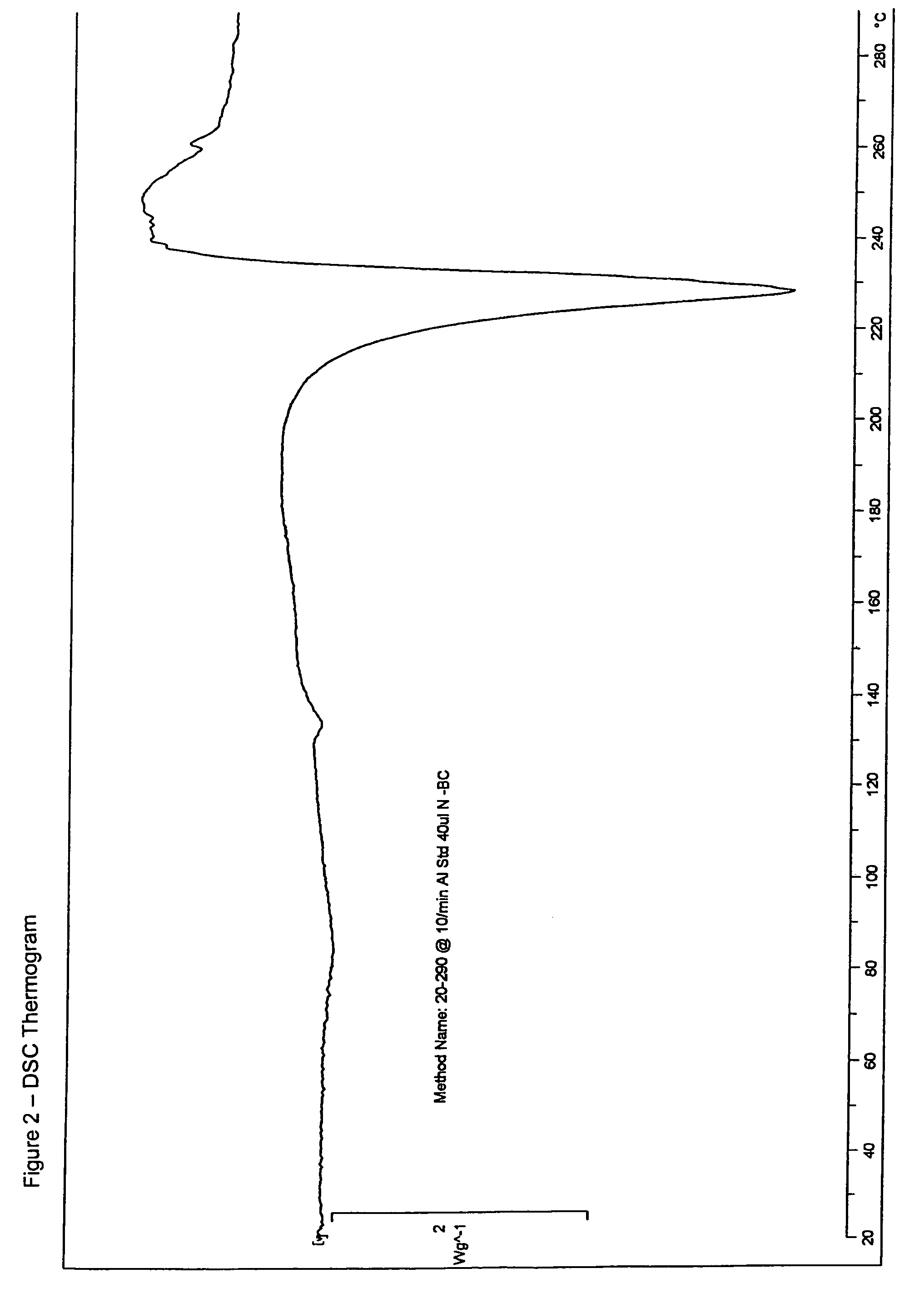
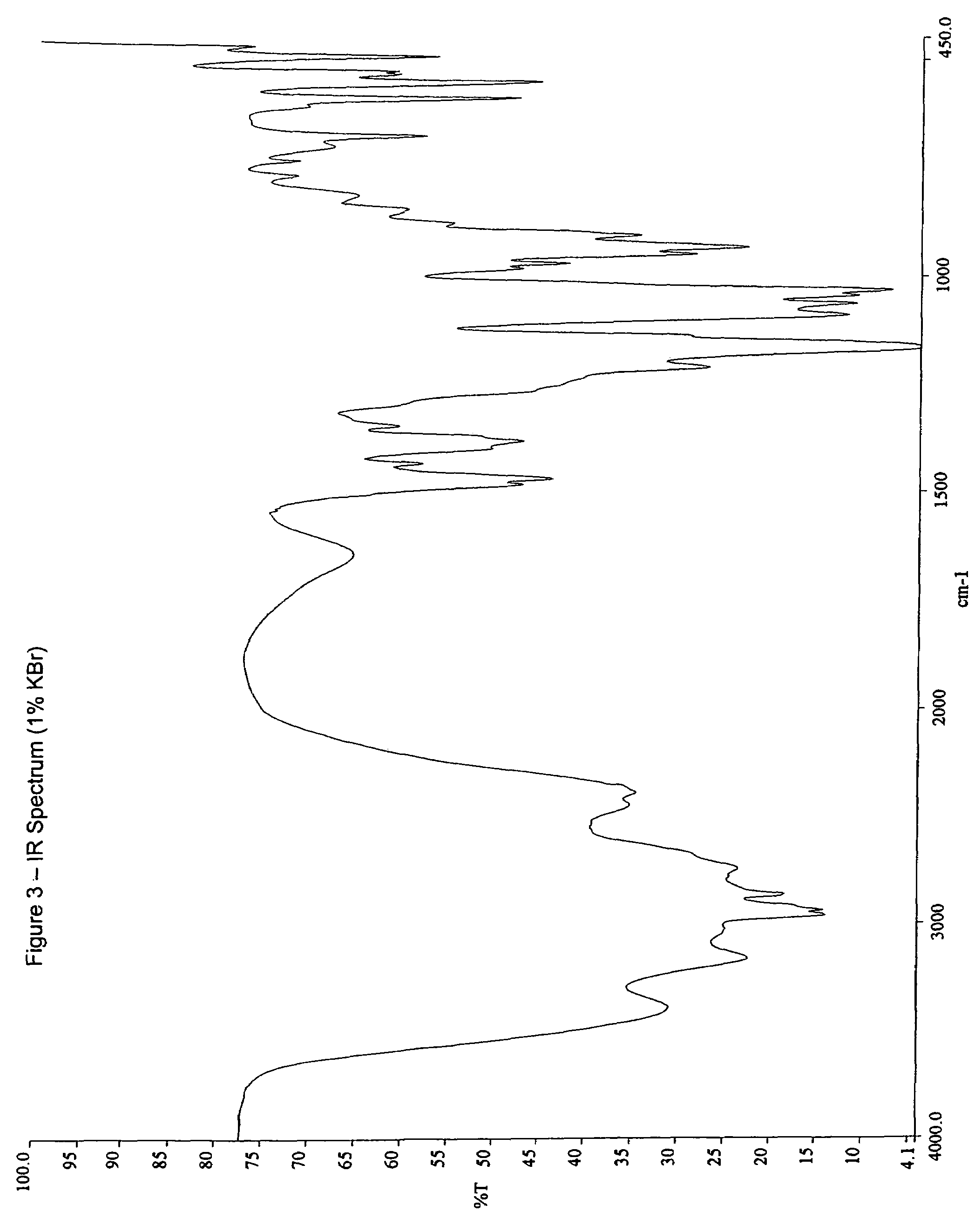
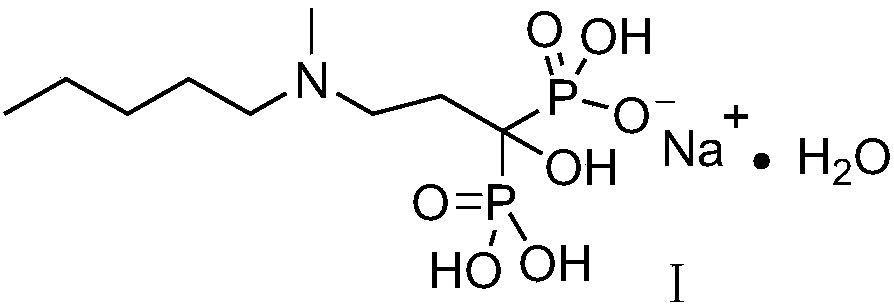
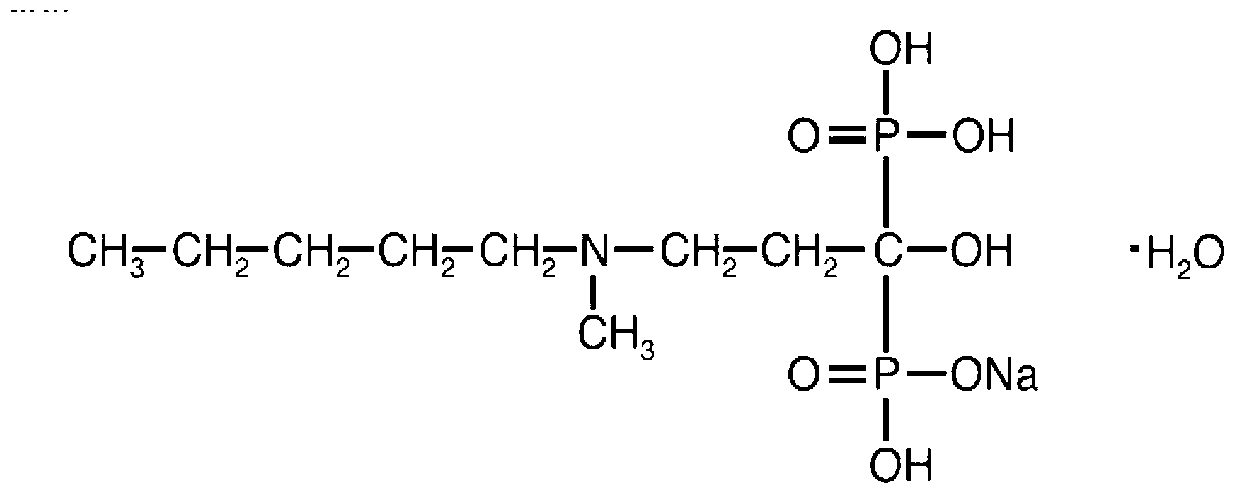Patents
Literature
47 results about "Ibandronate Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
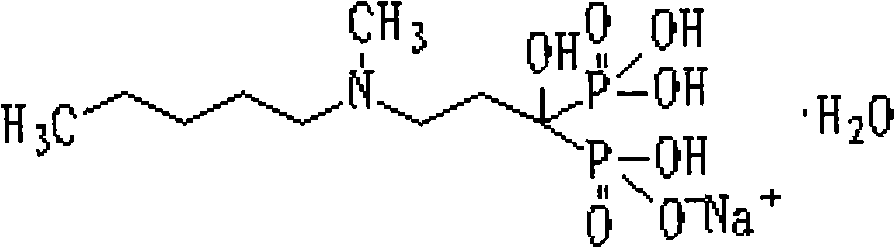
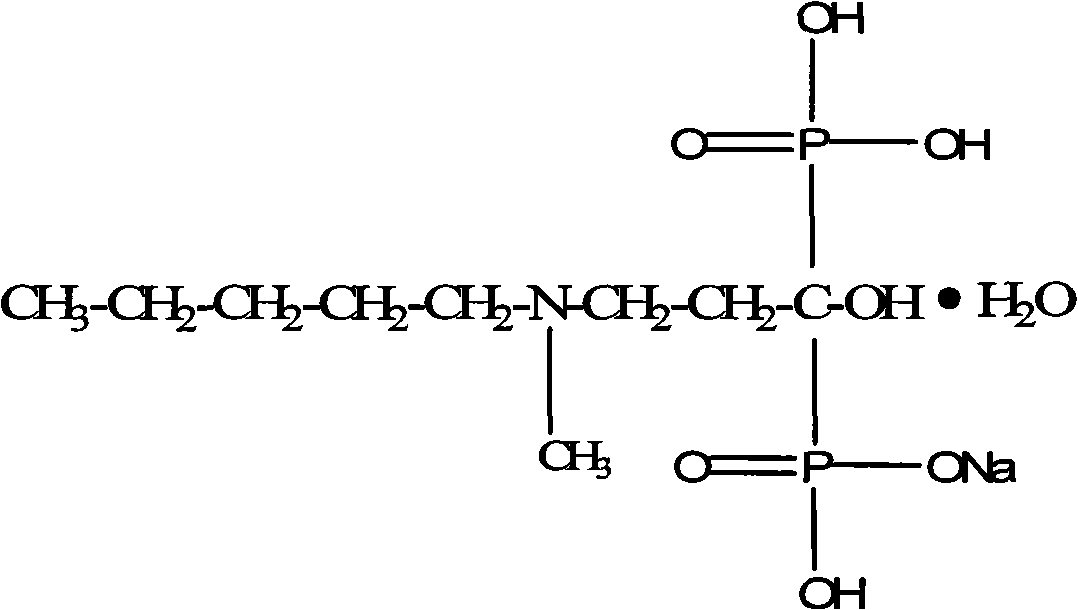
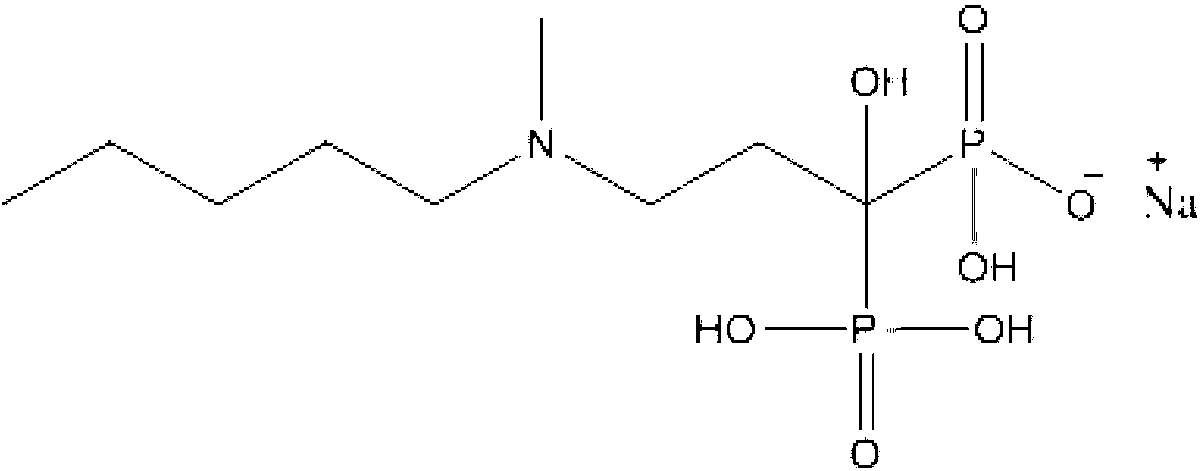
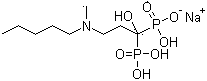
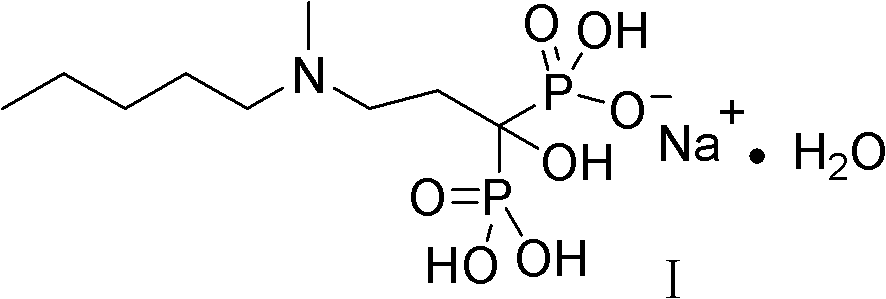
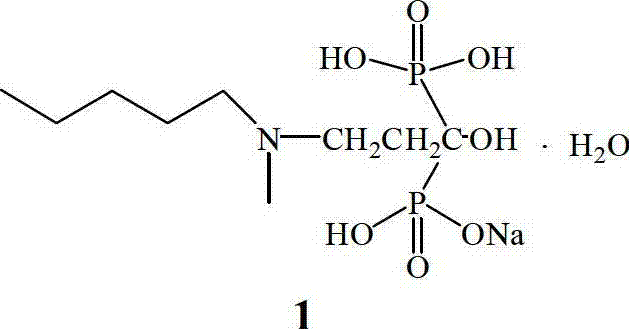
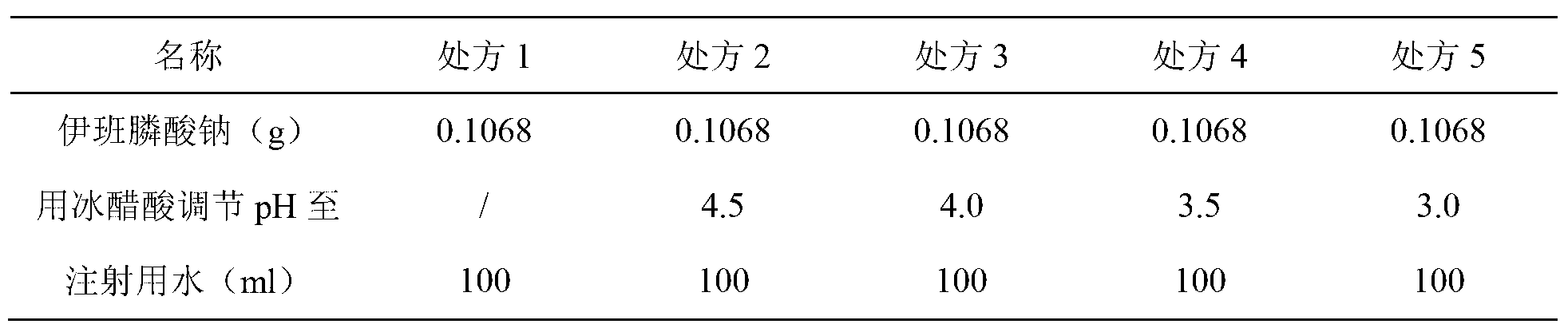
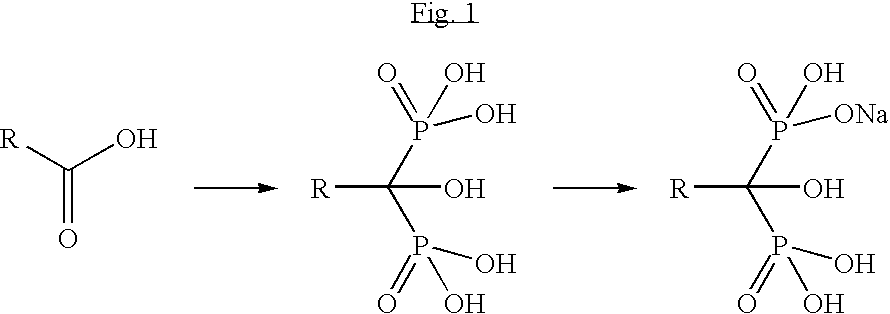
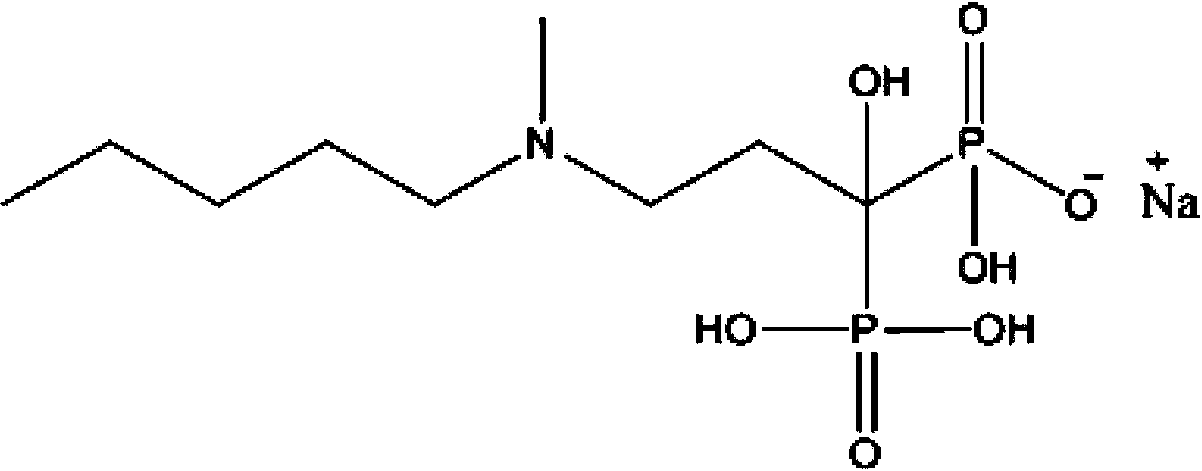
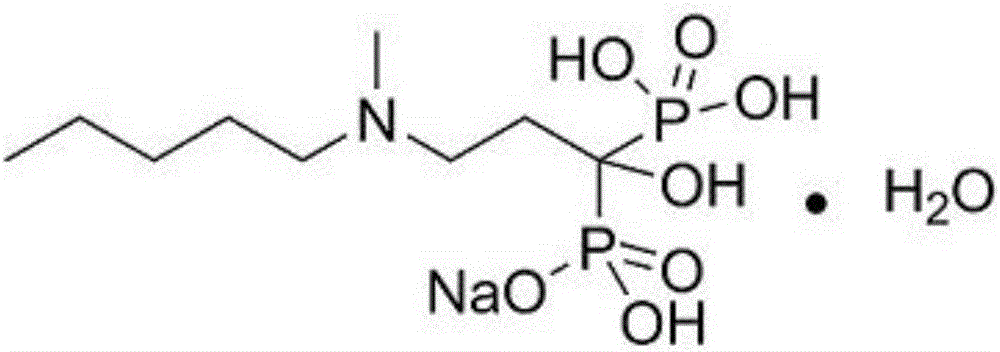
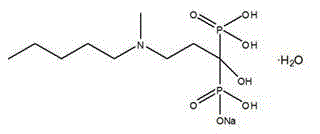
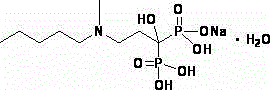
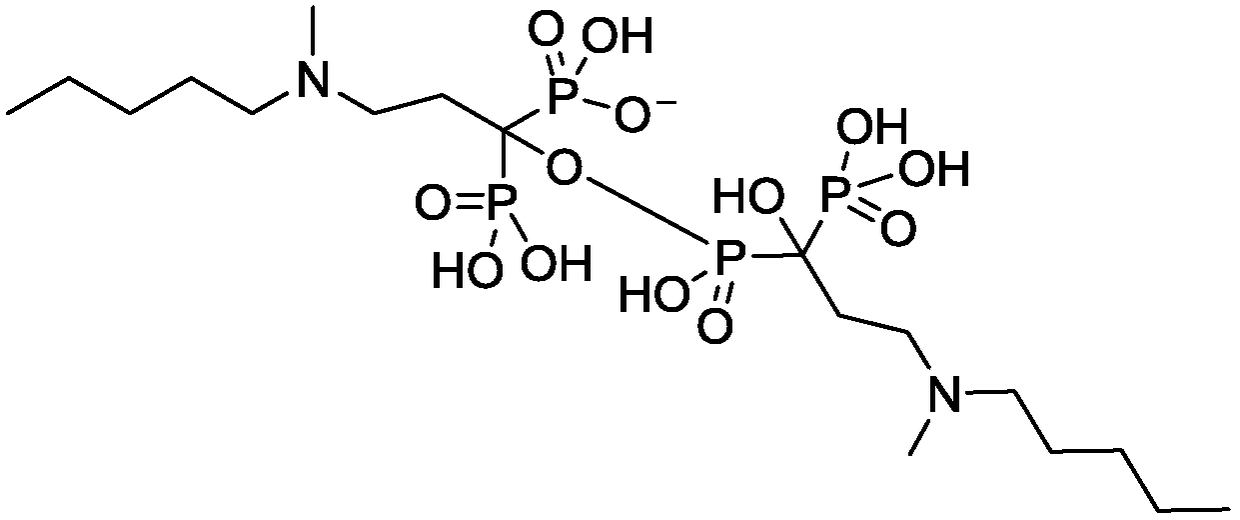
The sodium salt form of ibandronic acid, a synthetic nitrogen-containing bisphosphonate. Ibandronic acid inhibits farnesyl pyrophosphate synthase, resulting in a reduction in geranylgeranyl GTPase signaling proteins and apoptosis of osteoclasts. This agent increases bone mineral density, decreases bone remodeling, inhibits osteoclast-mediated bone resorption, and reduces metastases-related and corticosteroid-related bone pain.
Medicine compounds for treating osteoporosis
ActiveCN101229177AReduce dosageSignificant effectOrganic active ingredientsSkeletal disorderMicro structureSide effect
The invention provides a medical compound for treating osteoporosis which is characterized in that the invention contains strontium ranelate and bisphosphonate. Animal experiments indicate that the invention achieves the unexpected effect for treating the osteoporosis. The osteoporosis is a bone disease of the whole body characterized by the low bone mass and the degeneration of the micro structure of the bone organization, companying with the enhancement of the bone fragility and easy happened bone broken for which no ideal treatment medicine exists in the clinic. The bisphosphonate of the invention comprises alendronate, risedronate sodium, ibandronate, pamidronate, Etidronate, disodium clodronate and zoledronic acid, etc. In the invention, the dosage of the bisphosphonate is greatly reduced, which can effectively reduce the happening of side effects and is convenient to use the medicine.
Owner:LUNAN PHARMA GROUP CORPORATION
Delivery of bisphosphonates by microinjection systems
A system for the administration of a bisphosphonate to a subject comprises a bisphosphonate formulation and a microinjection device. In an embodiment, the bisphosphonate formulation includes ibandronate sodium. In some cases, the bisphosphonate formulation includes an excipient. The bisphosphonate formulation may be delivered to the subject subcutaneously, transdermally or intradermally.
Owner:LANCO BIOSCI
Preparation method of compound sodium ibandronate
InactiveCN101863919AReduce usageReduce the number of refinementsGroup 5/15 element organic compoundsPropanoic acidIbandronate Sodium
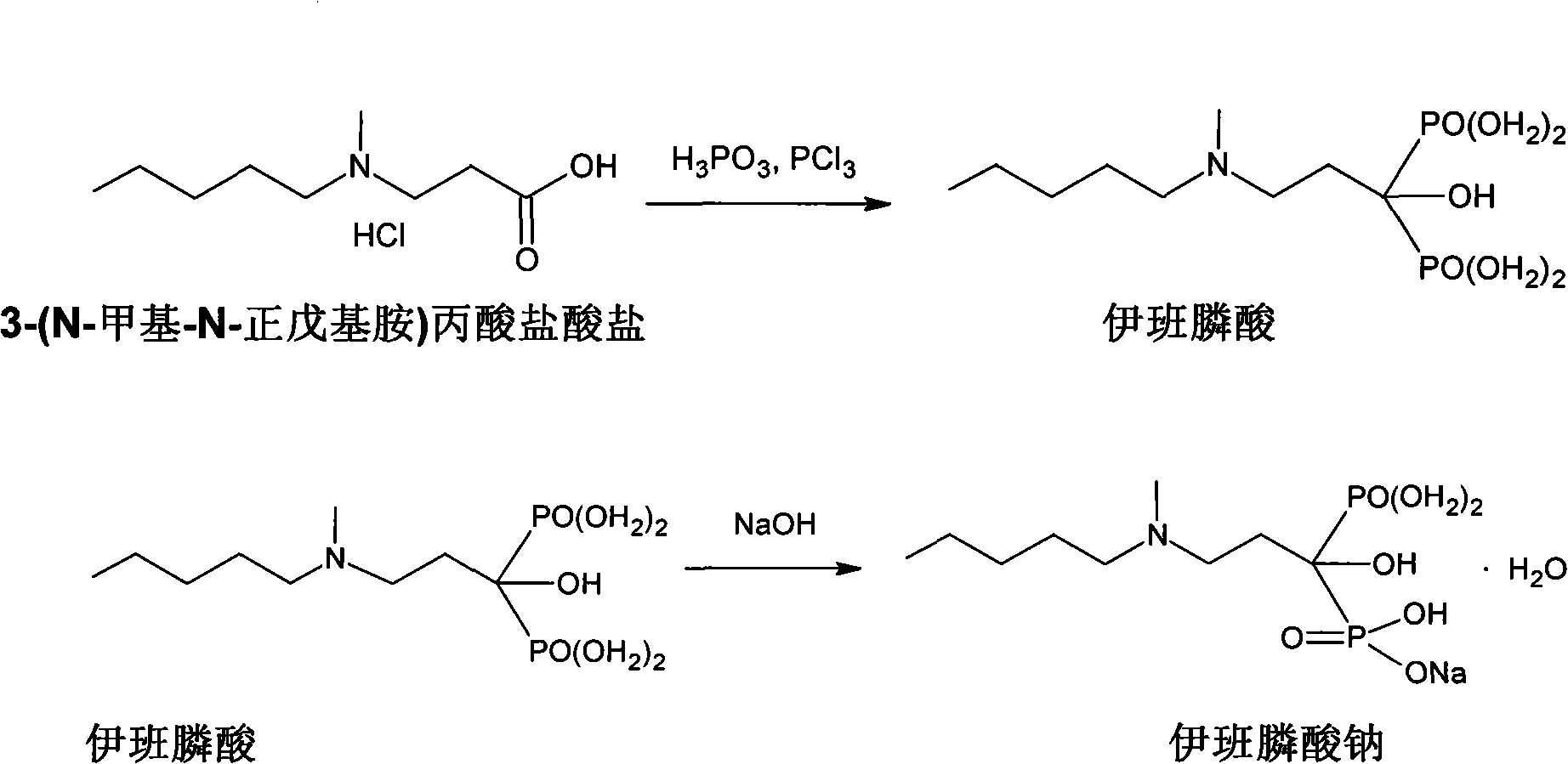
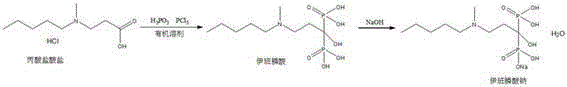
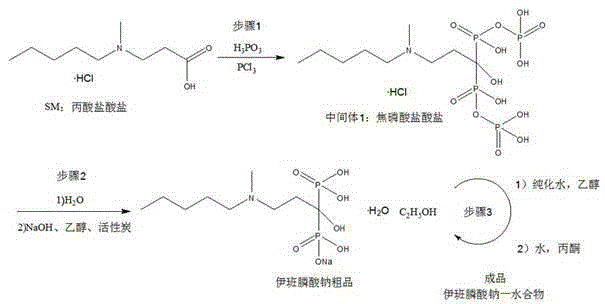
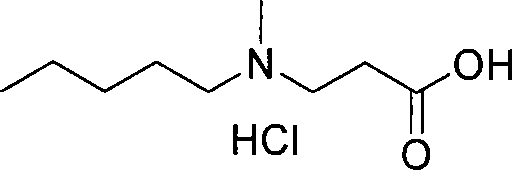
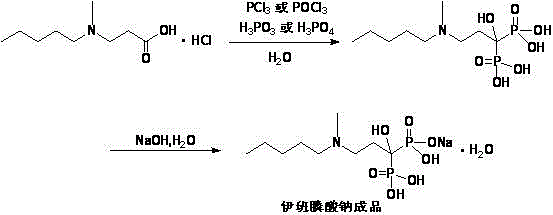
The invention relates to a synthetic method of sodium ibandronate, which comprises the following steps: adding 3-(N-methyl-N-n-amyl amine)propanoic acid hydrochloride, orthophosphorous acid and phosphorus trichloride into a solvent, reacting to obtain ibandronic acid, and forming the salt of the ibandronic acid and sodium hydroxide to obtain the sodium ibandronate, and is characterized in that the added solvent is acetonitrile, the acetonitrile is used as the solvent, and a reaction product can be uniformly dispersed in the solvent, thereby the defect that other solvents enable the product to be in the shape of oil blocks to enable stirring to be more difficult is overcome, so that the process can be successfully used for large-scale production; the mol ratio of the 3-(N-methyl-N-n-amyl amine)propanoic acid hydrochloride to the phosphorus trichloride is 1:1.20 to 1.39, and the usage amount of the phosphorus trichloride is reduced, so that the refinement times can be reduced, thereby the refinement loss is reduced.
Owner:武汉同源药业有限公司
Synthetic method of ibandronate
InactiveCN101279985AHigh yieldFew reaction stepsGroup 5/15 element organic compoundsSkeletal disorderPropanoic acidPropionitrile
The invention discloses a method to synthesize ibandronate, aiming to simplify process and improve yield. The method includes the following steps: 1, producing crude 3-(N-methyl-N-n-amyl-amine)propionitrile liquid; 2, producing 3-(N-methyl-N-n-amyl-amine)propionic acid hydrochloride; 3, producing white crude ibandronate. Compared with the prior art, the method has less procedure and simple after-process and effectively improves total yield. Therefore, the method has high yield and low cost, and the operation is simple, safe and convenient for industrial production.
Owner:HANDE PHARMA
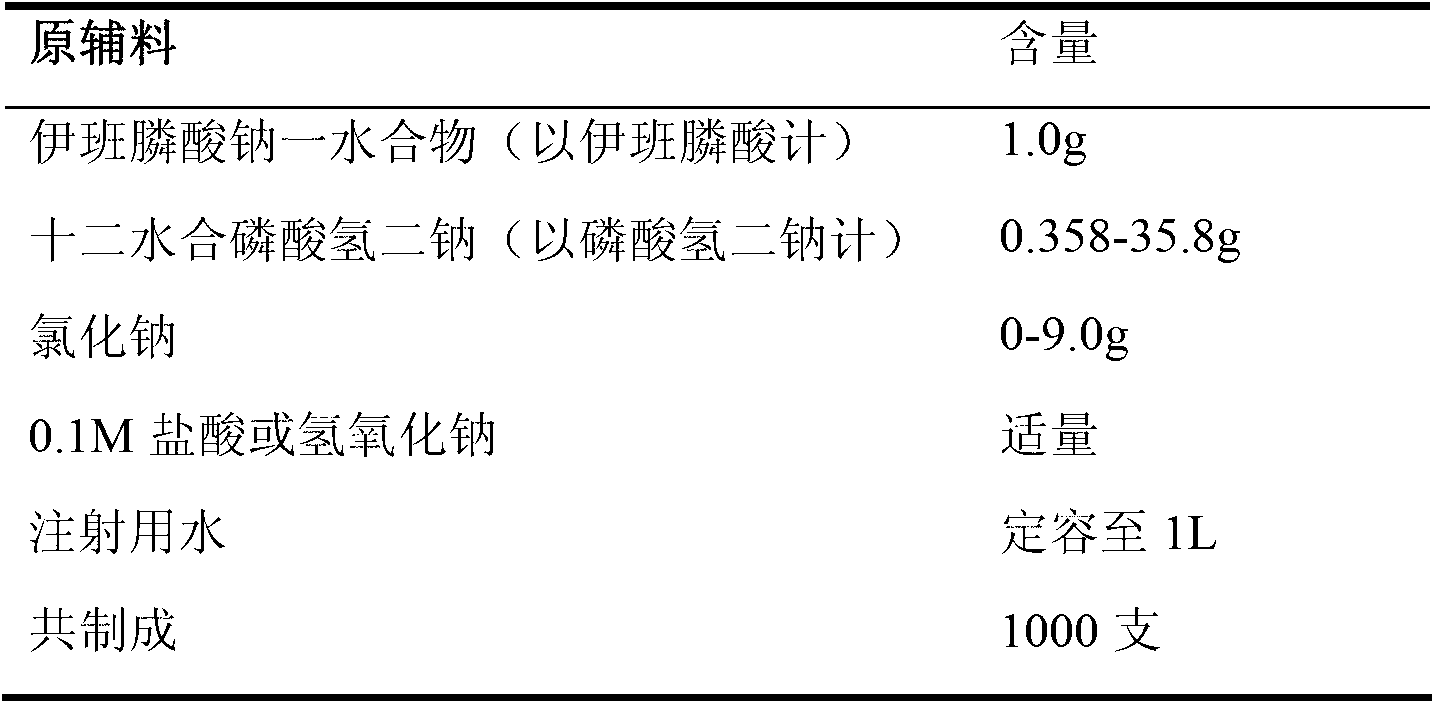
Ibandronate sodium containing injection
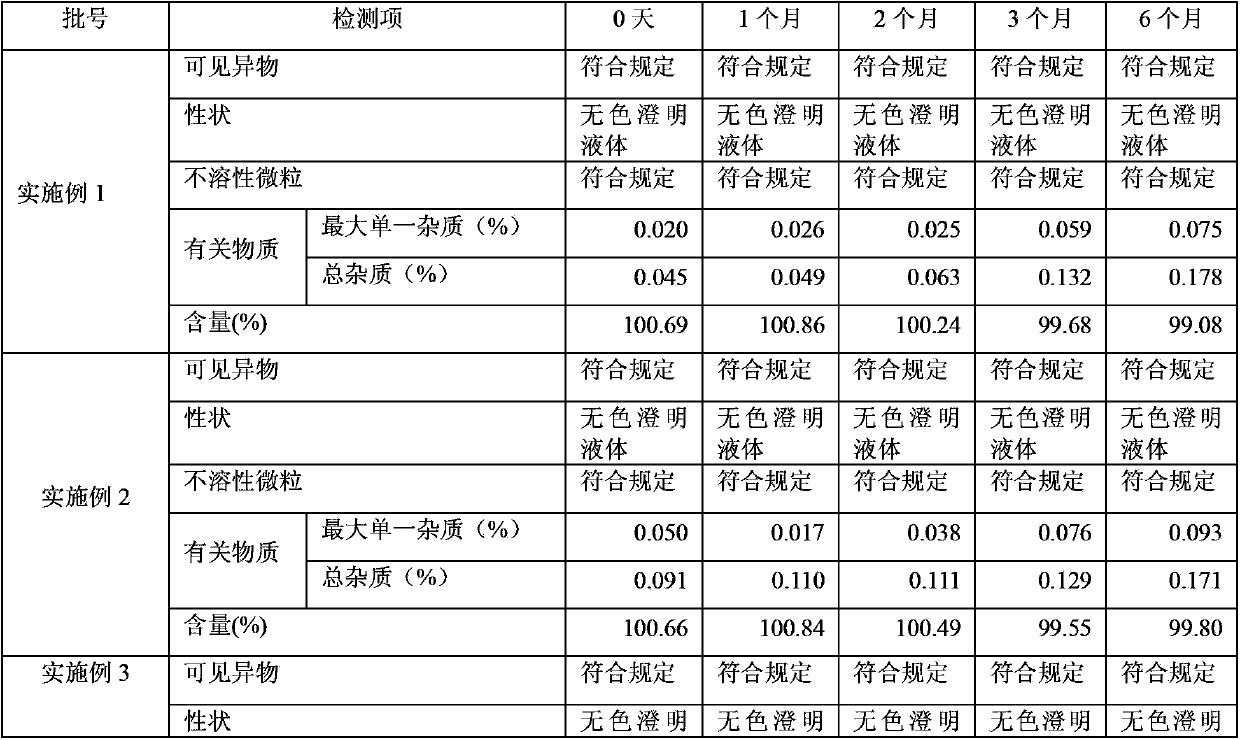
ActiveCN103070824AStable main drug contentImprove stabilityOrganic active ingredientsInorganic non-active ingredientsActivated carbonPhosphate
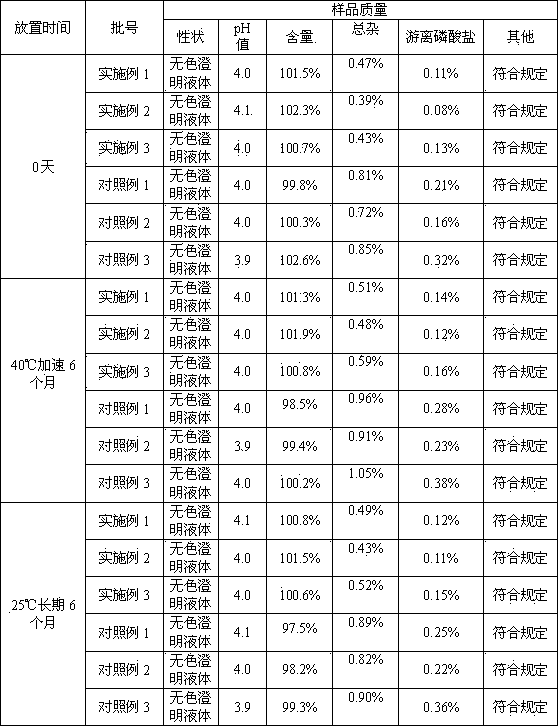
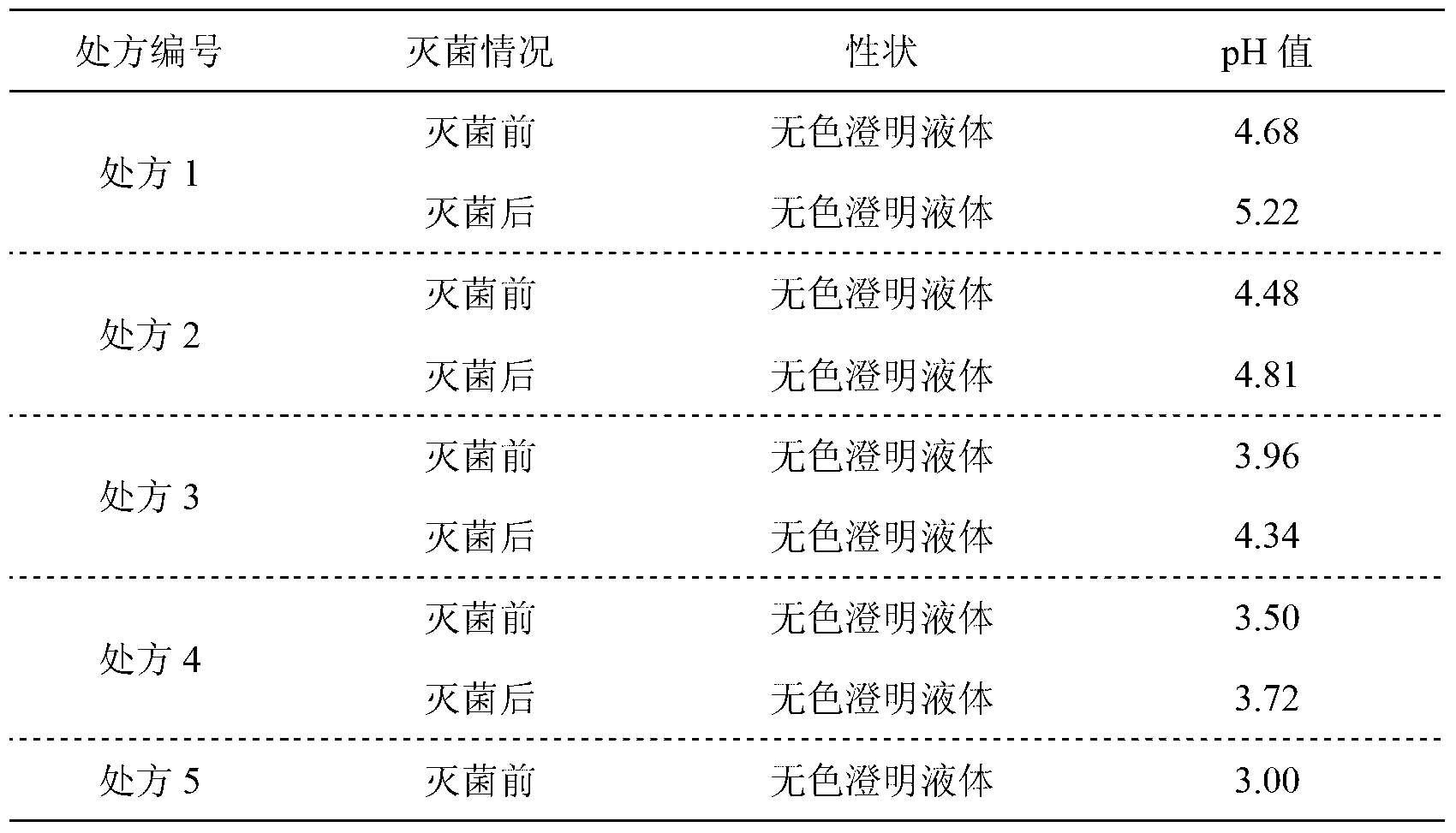
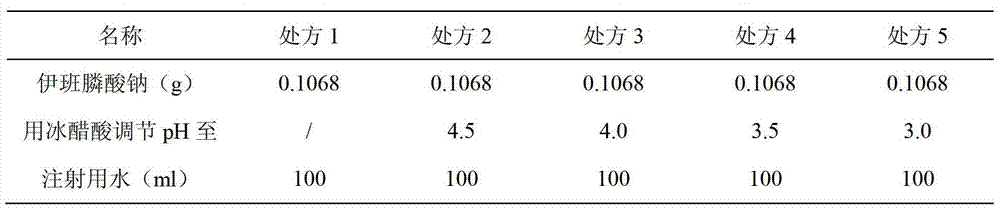
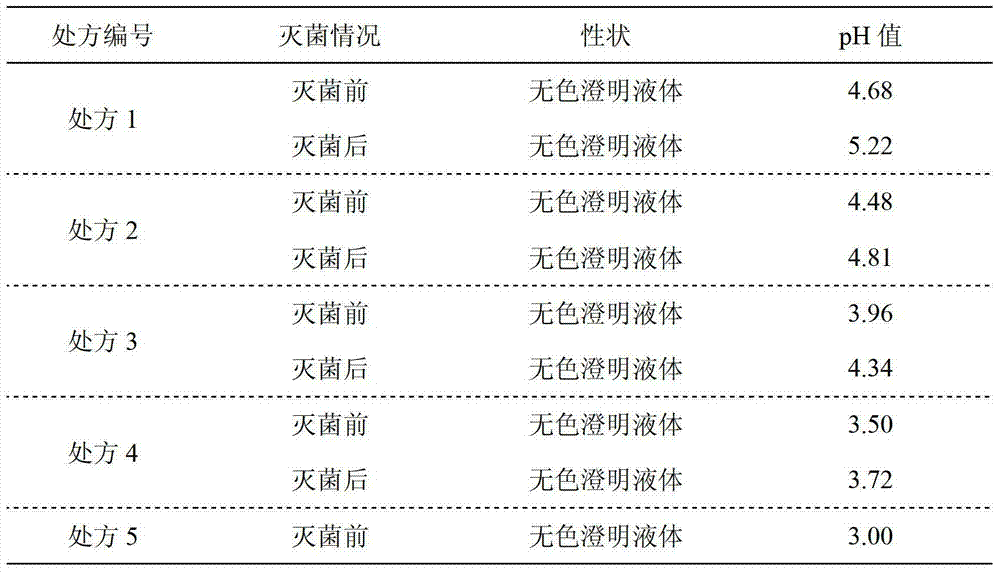
The invention relates to an ibandronate sodium containing injection. The injection is prepared by ibandronate sodium and pharmaceutically acceptable auxiliary materials; a pH value of the injection is controlled to be 3.5-4.5 by a phosphate buffer; activated carbon accounting for 0.1% of the total volume of the injection is used for removing a pyrogen; and adsorption of activated carbon to a main medicine is avoided while the pyrogen is removed. An accelerated stability test indicates that the impurity content of the injection is lower than that of the marketed injection, and the quality is stable and controllable.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Method for detecting related substances in sodium ibandronate injection
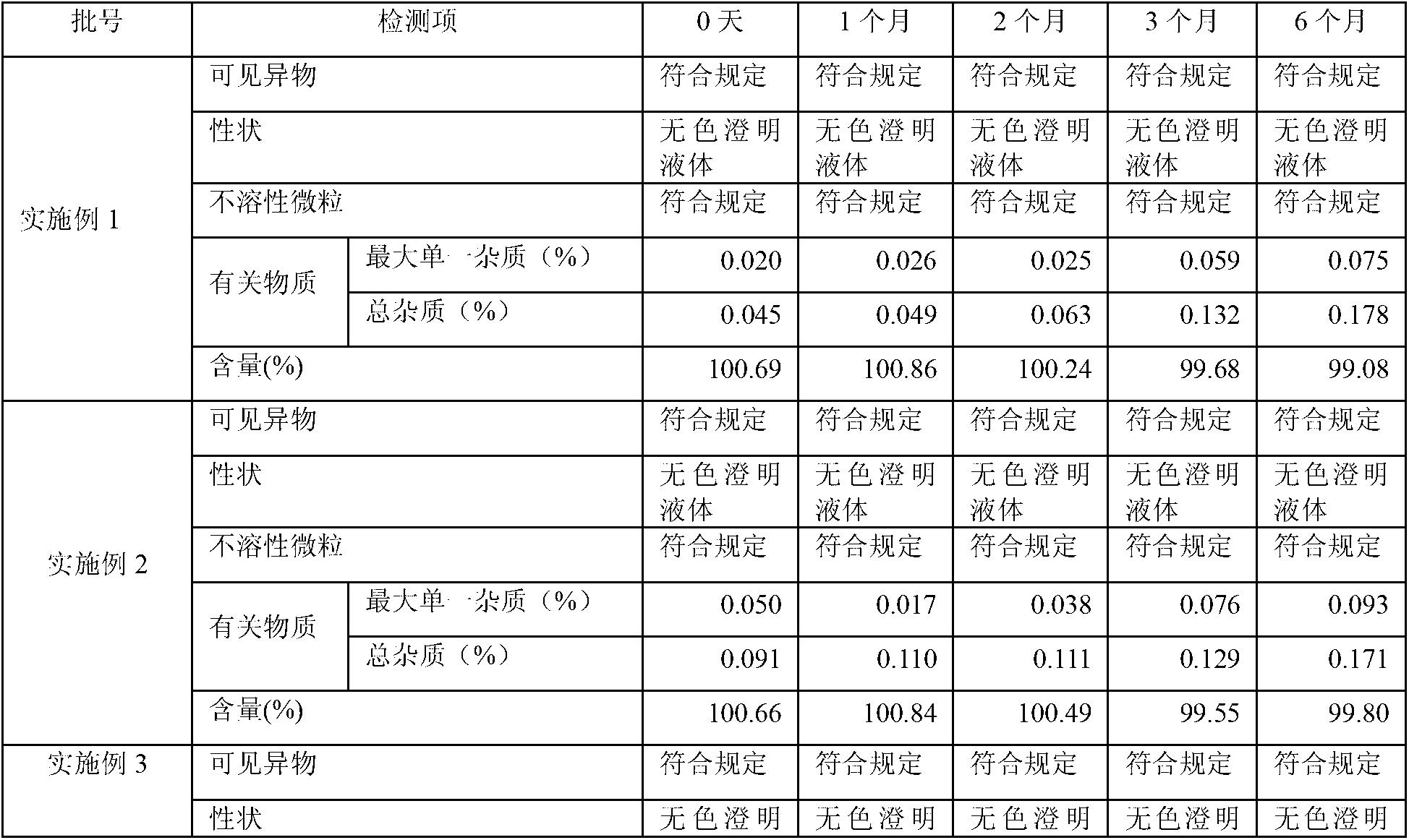
InactiveCN109668975AOptimal pre-processing methodEnsure safetyComponent separationSilanesCharged aerosol detector
The invention provides a method for detecting related substances in sodium ibandronate injection, and belongs to the technical field of drug analysis. According to the method for detecting the relatedsubstances in the sodium ibandronate injection, a high performance liquid chromatograph-charged aerosol detector (CAD) is adopted, a chromatographic column with octadecyl silane chemically bonded silica embedded in a strong anion exchange group as a filler is adopted, acetonitrile-water-trifluoroacetic acid serves as a flowing phase for gradient elution, the injection is subjected to Ag / H type pretreatment small column treatment, and thus the related substances in the sodium ibandronate injection can be effectively separated and determined.
Owner:QILU PHARMA
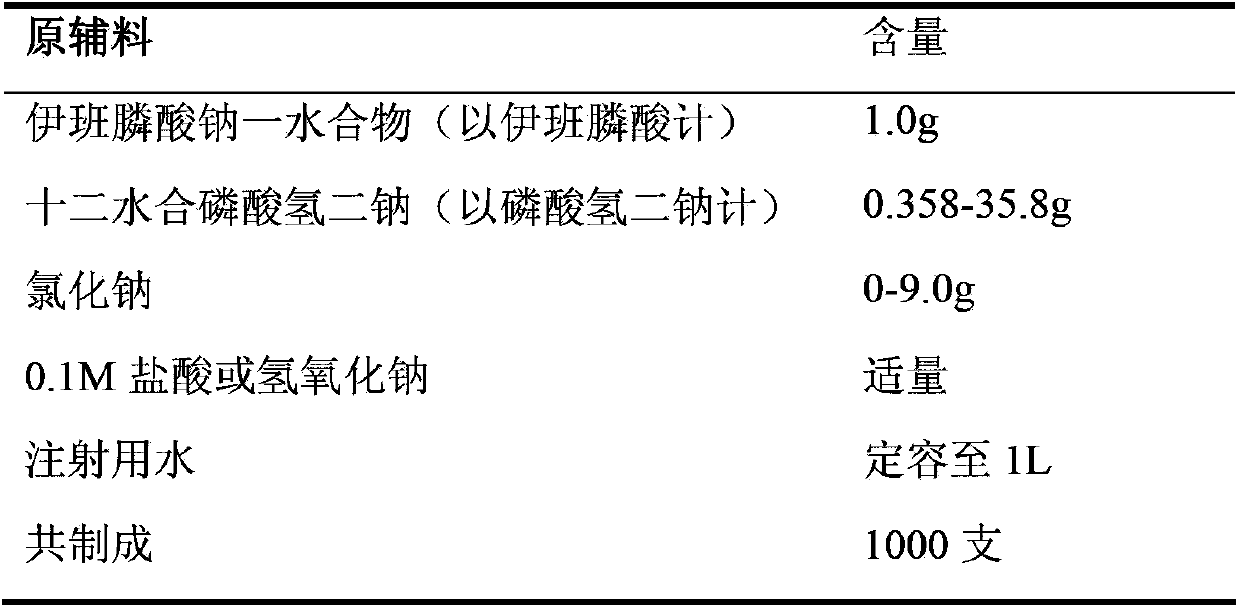
Sodium ibandronate injection composition
InactiveCN103385882ALow Free Phosphate ContentSensitive detectionOrganic active ingredientsMetabolism disorderPharmacyIbandronate Sodium
The invention belongs to the technical field of medicines, especially relates to the field of pharmacy, and specifically relates to a sodium ibandronate injection composition. The composition consists of sodium ibandronate, an osmotic-pressure conditioning agent, an antioxidant, a pH conditioning agent and injection water, wherein the content of free phosphate is less than 0.2%. The sodium ibandronate injection composition not only is accord with a quality standard, but also is more stable, safer for patients, and more suitable for clinical application. Additionally, the invention also provides a detection method for an extremely low concentration phosphate; and the method is high in sensitivity, is capable of more accurately detecting the content of the free phosphate in the sodium ibandronate injection composition, and guaranteeing sensitivity and accuracy of quality detection on the sodium ibandronate injection composition.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
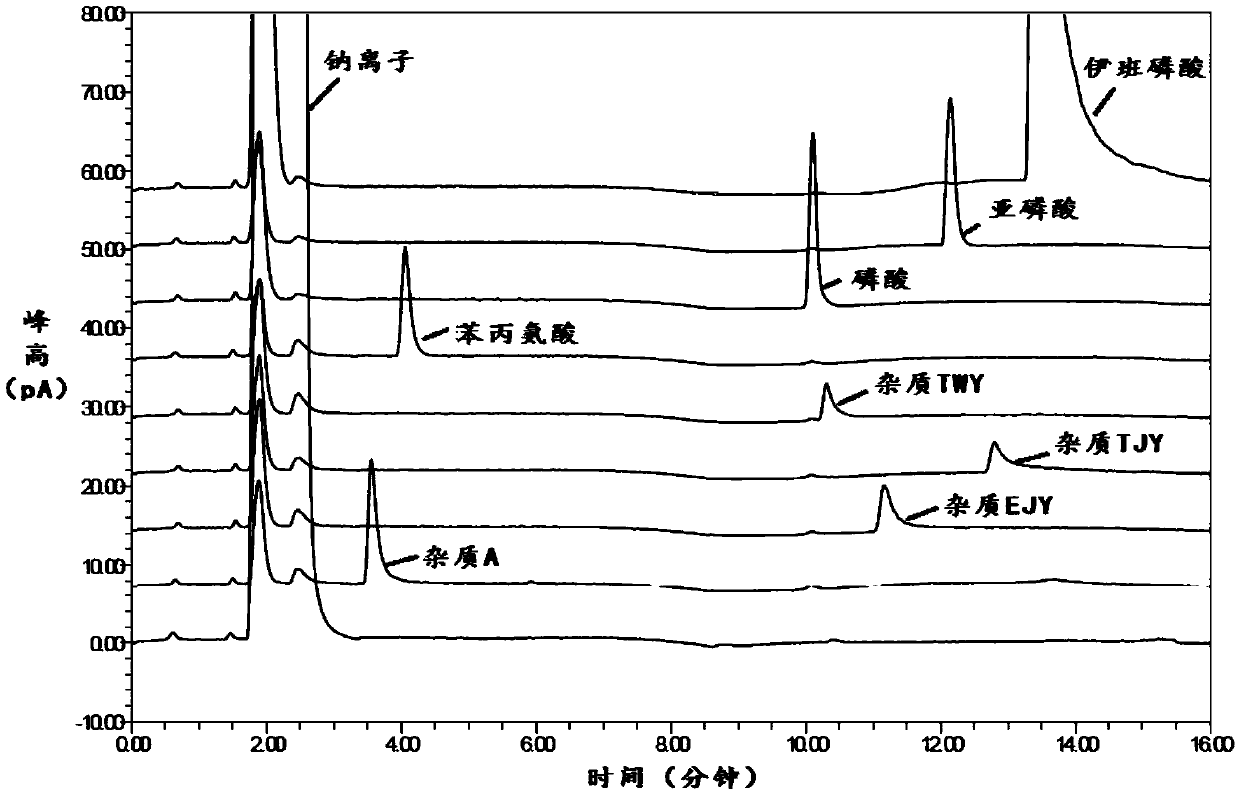
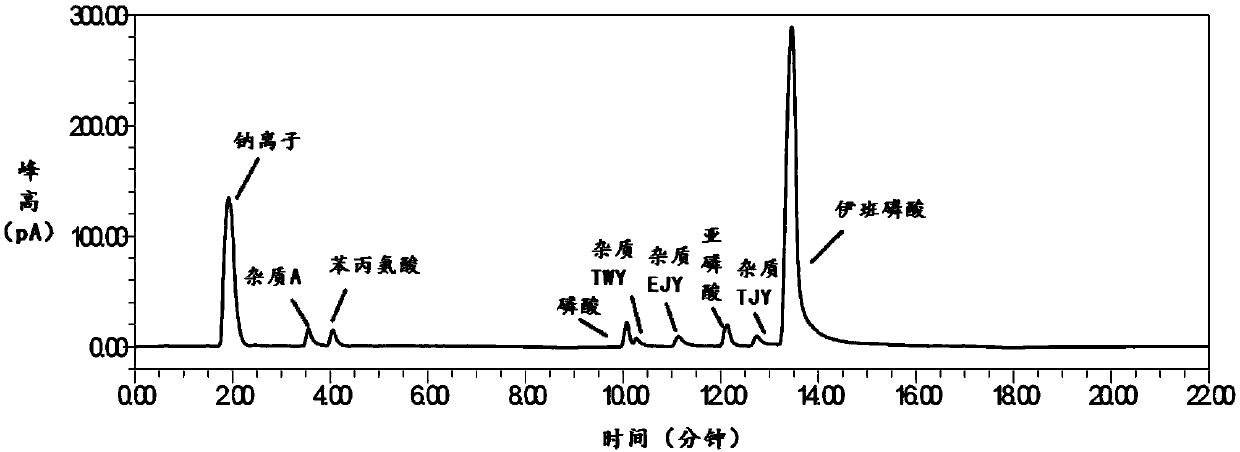
Method for analytical separation sodium ibandronate and impurities thereof by utilizing high performance liquid chromatography (HPLC)
InactiveCN103134886AEffective controlSolve the problem of effective separationComponent separationUltraviolet detectorsPhosphate
The invention discloses a high performance liquid chromatography (HPLC), in particular to a method for analyzing sodium ibandronate and pereparation by utilizing the HPLC. Octyl or octadecyl silicane bonded silica gel is adopted as a chromatographic column of filler, methyl alcohol-phosphate buffered saline or acetonitrile-phosphate buffered saline is used as a mobile phase for chromatographic column separation, and an ultraviolet detector is used for detecting the sodium ibandronate and the impurities of the sodium ibandronat. With the method, effective separation of the sodium ibandronat, the impurities I and the impurities II can be conducted well, the contents of the sodium ibandronat can be measured accurately, and quality of sodium ibandronat products can be guaranteed well. The method has the advantages of being strong in specificity, simple in operation and high in accuracy.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
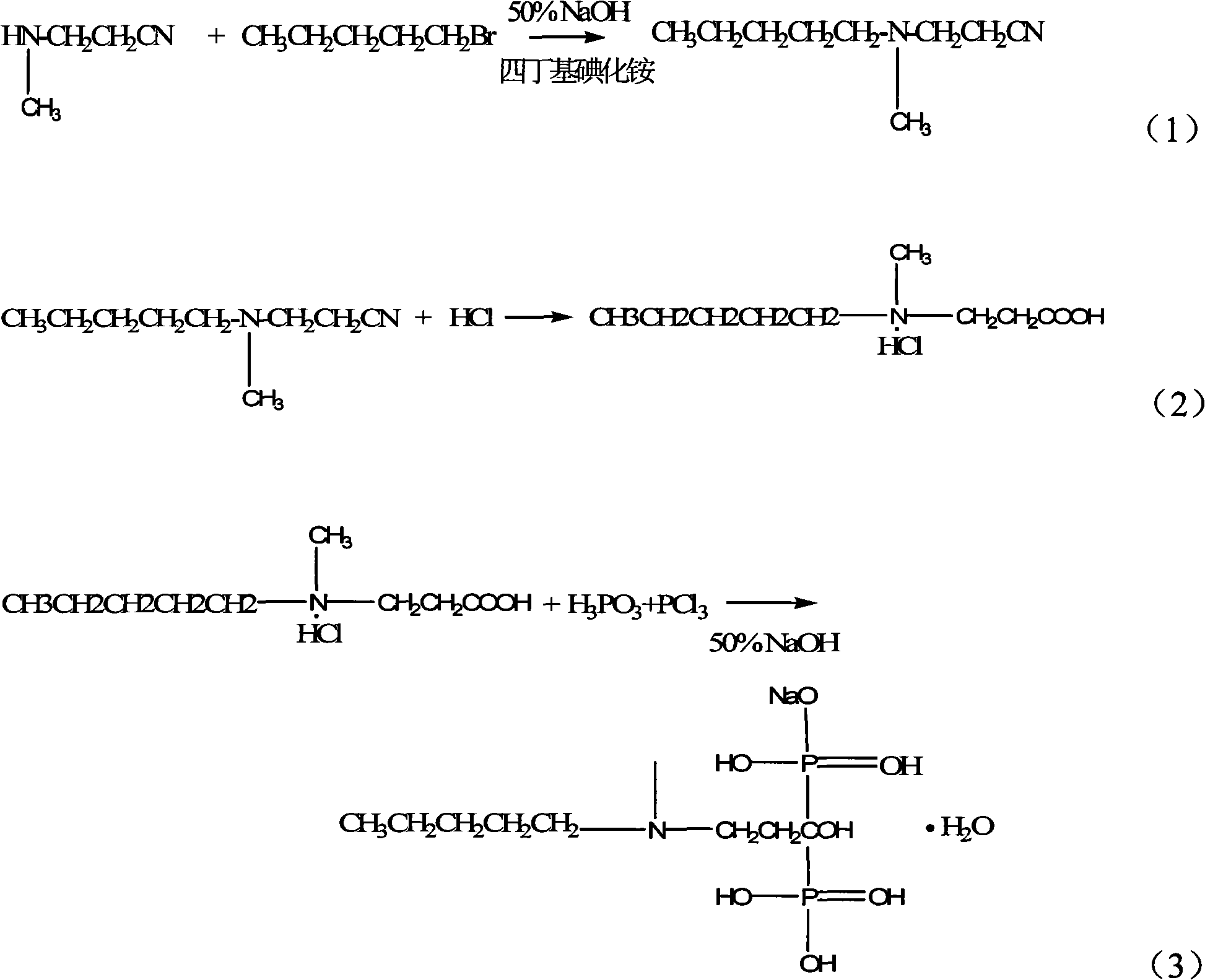
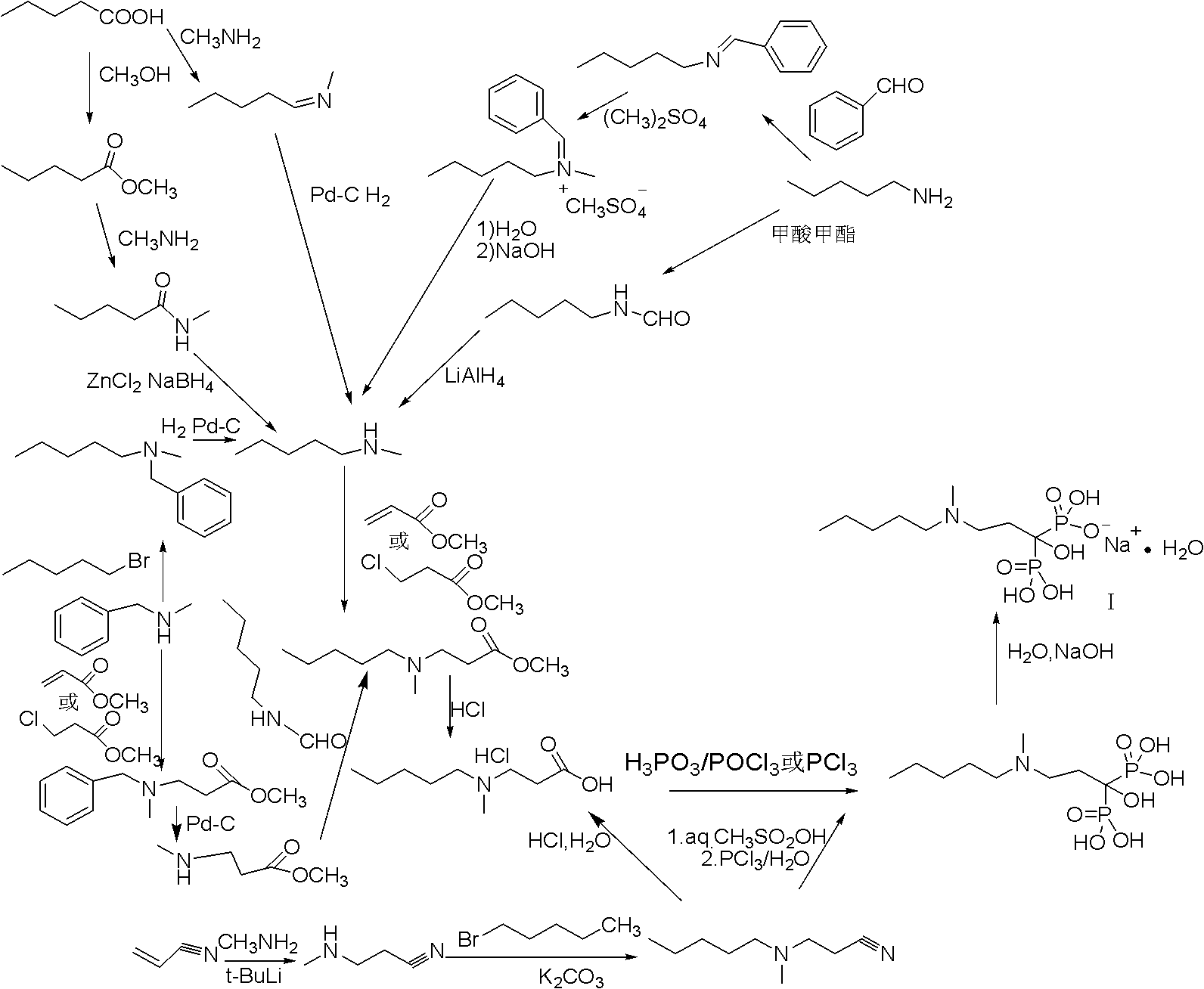
Method for synthesizing sodium ibandronate
ActiveCN102093416AFew stepsEasy to operateGroup 5/15 element organic compoundsIbandronate SodiumReaction step
The invention discloses a method for synthesizing sodium ibandronate. The sodium ibandronate is prepared by the following steps: reacting an intermediate compound (III) with halogenated n-pentane; and then hydrolyzing and acidifying so as to obtain the sodium ibandronate, wherein in the formula (III), R1 is C1-4 alkyl. The method has the characteristics that the reaction steps are less, the used reagent is small in toxicity, the safety is good, and the like.
Owner:南京恒生制药有限公司 +1
Method for preparing sodium ibandronate
ActiveCN103030661AHigh purityHigh reaction yieldGroup 5/15 element organic compoundsPhosphorous acidN dimethylformamide
The invention provides a method for preparing sodium ibandronate. The method comprises the following steps of: performing amyl reaction on 3-methylamino propionitrile and n-bromopentane in an N,N-dimethylformamide solution of anhydrous potassium carbonate to obtain 3-(N-methyl-N-amyl) aminopropionitrile; performing hydrolysis reaction on the 3-(N-methyl-N-amyl) aminopropionitrile and a phase transfer catalyst under an alkaline condition to obtain 3-(N-methyl-N-amyl) aminopropionic acid; and performing diphosphonic acidification and hydrolysis reaction on the 3-(N-methyl-N-amyl) aminopropionic acid, phosphorous acid and thionyl chloride in the presence of toluene to obtain ibandronic acid; and performing neutralization reaction on the ibandronic acid under an alkaline condition to obtain the sodium ibandronate. By adopting the method, the technical problems of low yield, low purity, more steps and high production cost of the sodium ibandronate in the prior art are solved.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
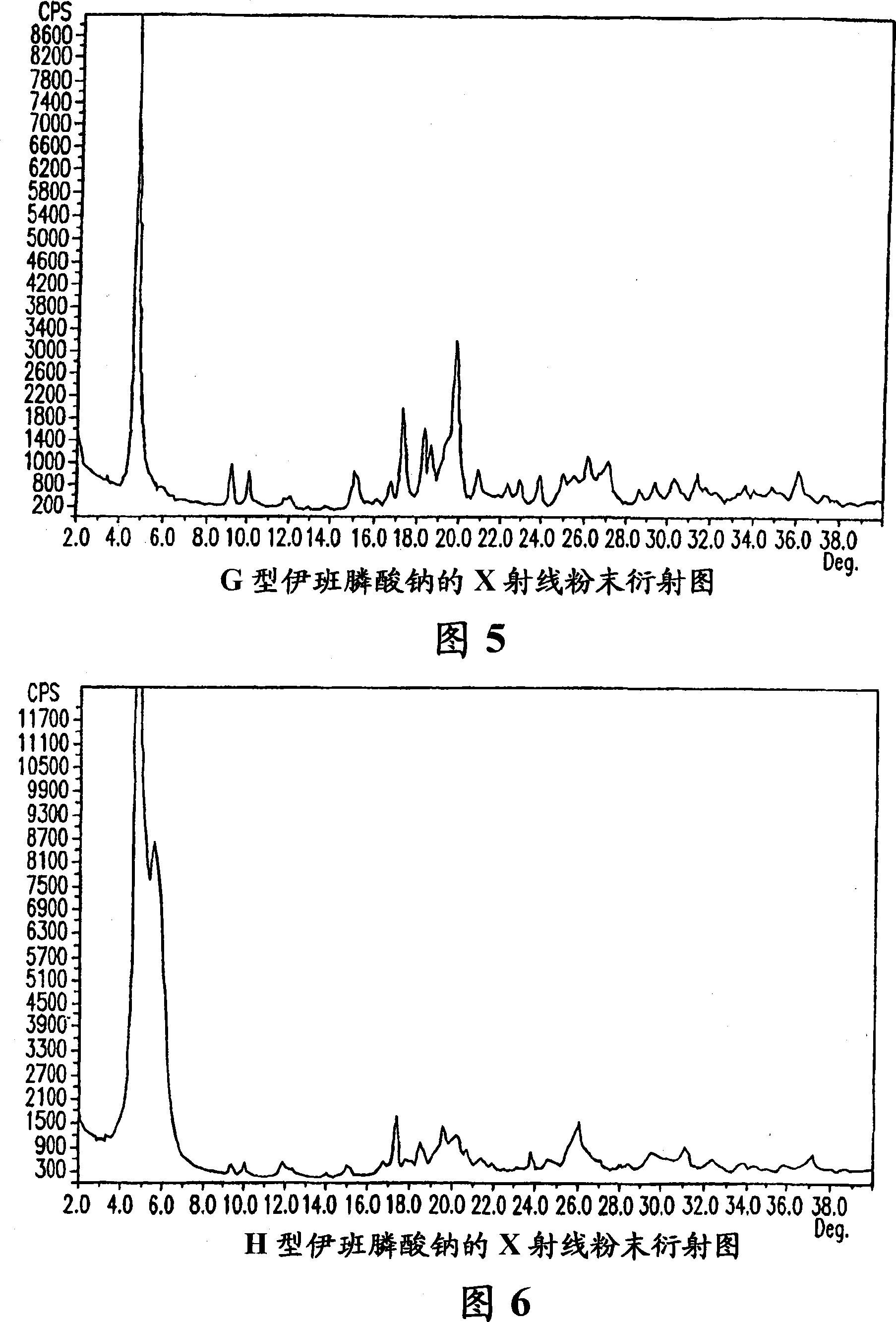
Solid and crystalline ibandronate sodium and processes for preparation thereof
InactiveCN101022812AOrganic active ingredientsMetabolism disorderIbandronate SodiumOrganic chemistry
Owner:TEVA PHARMA IND LTD
Sodium ibandronate injection composition
ActiveCN104922060ASimple prescriptionPrescription validOrganic active ingredientsPharmaceutical delivery mechanismIbandronate SodiumBULK ACTIVE INGREDIENT
The invention provides a sodium ibandronate injection composition. A sodium ibandronate injection comprises sodium ibandronate serving as an active ingredient, glucose serving as a stabilizer and water for injection. The sodium ibandronate injection has the characteristics of simple prescription, high stability, high safety, suitability for industrial production and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Sodium-ibandronate-loaded PLGA microspheres and method for preparing complex-tissue engineering bone employing microspheres
PendingCN113116858AHigh drug loading rateImprove material propertiesOrganic active ingredientsSkeletal disorderMicrosphereIbandronate Sodium
The invention relates to the technical field of pharmacochemistry and discloses sodium-ibandronate-loaded PLGA microspheres and a method for preparing complex-tissue engineering bone employing microspheres. The preparation method applying a re-emulsification solvent evaporation method comprises the following steps: dissolving sodium-ibandronate-loaded PLGA into a dichloromethane solution, carrying out mixing through vortex stirring to form a uniform solution, transferring the mixed solution into a beaker containing a PVA aqueous solution, carrying out stirring so as to evaporate the solvent and stabilize a microsphere form, and washing microsphere forms thrice with distilled water, so as to separate and obtain the sodium-ibandronate-loaded PLGA microspheres. According to the microspheres and the preparation method, the sodium ibandronate / PLGA / CPC-loaded complex-tissue engineering bone has good biocompatibility and osteogenesis activity, the adhesion, proliferation and osteogenesis differentiation of mesenchymal stem cells of bone marrow can be promoted, the formation of local new bone of necrotic femoral head is facilitated, the repairing and healing of the necrotic femoral head are accelerated, and thus, the complex-tissue engineering bone can be applied to treatment on ischemic necrosis of the femoral head.
Owner:张皓轩
Sodium ibandronate injection composition and preparation method of sodium ibandronate injection composition
ActiveCN103239396ASimple prescriptionReduce contentOrganic active ingredientsMetabolism disorderAcetic acidSodium acetate
The invention provides a sodium ibandronate injection composition. Every 1000 injections consist of the following components: 1.0684-6.4101g of sodium ibandronate, 1.66-9.96g of sodium acetate, suitable amount of glacial acetic acid, and 1000-6000ml of water for injection. The sodium ibandronate injection prepared by the invention has the advantages that the prescription is simple, auxiliary materials are all commonly used by the injection, and the safety is high. Simultaneously the sodium acetate content is low, irritation cannot be produced in intravenous administration, the prepared final product is stable in quality, and the indexes of related substances and contents are all superior to those in the prior art.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Preparation method of sodium ibandronate monohydrate
InactiveCN104277070AAvoid genotoxic substancesImprove protectionGroup 5/15 element organic compoundsPhosphorous acidPropanoic acid
The invention discloses a preparation method of sodium ibandronate monohydrate. The method is characterized by comprising the following steps: adding 3-(N-methyl-n-pentylamine) propionic acid hydrochloride, phosphorous acid and phosphorus trichloride to solvent methylbenzene to react, so as to obtain an ibandronic acid ester; hydrolyzing to obtain ibandronic acid and salifying with sodium hydroxide; and after refining, adding water to concentrate, so as to obtain sodium ibandronate monohydrate. According to the process, genotoxic substances which can be generated can be well avoided, and the yield is high. By adoption of a water concentration method, the problems of patent protection and solvation can be well avoided, so as to obtain stable monohydrate.
Owner:湖北华世通生物医药科技有限公司
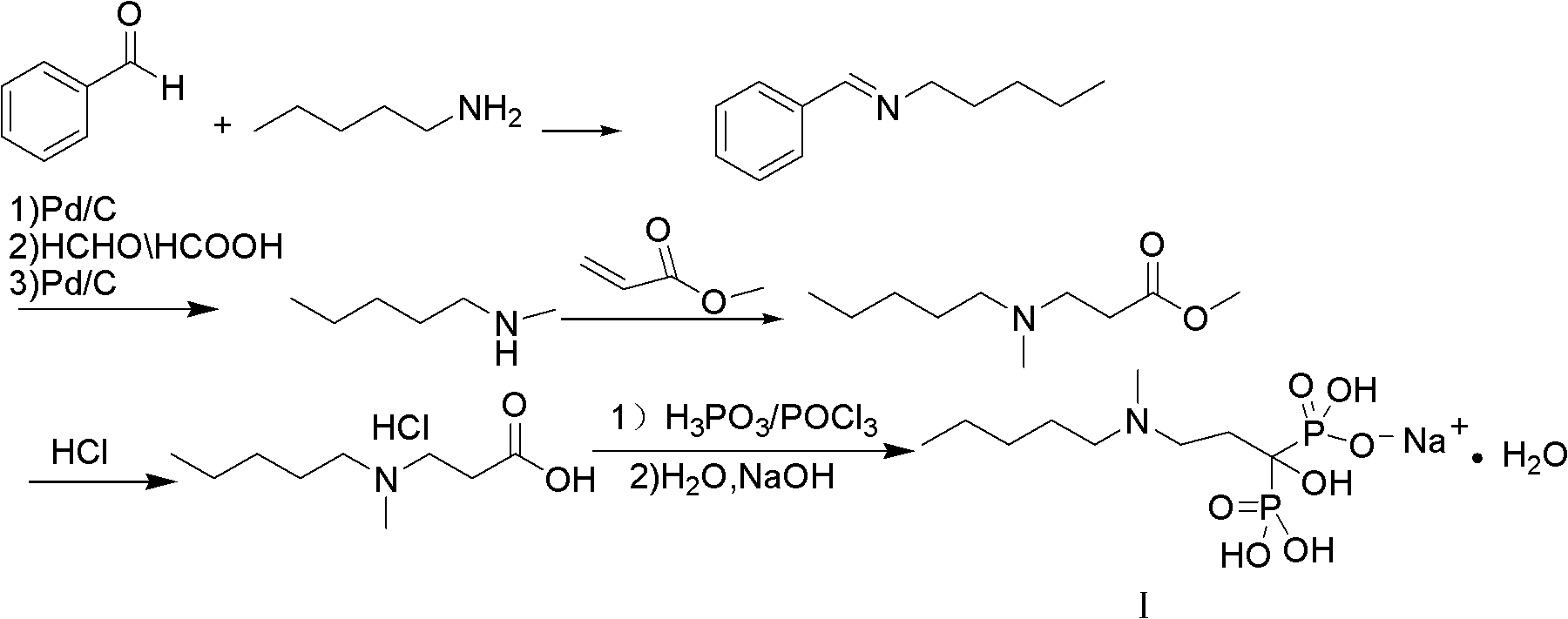
Process for the synthesis of ibandronate sodium
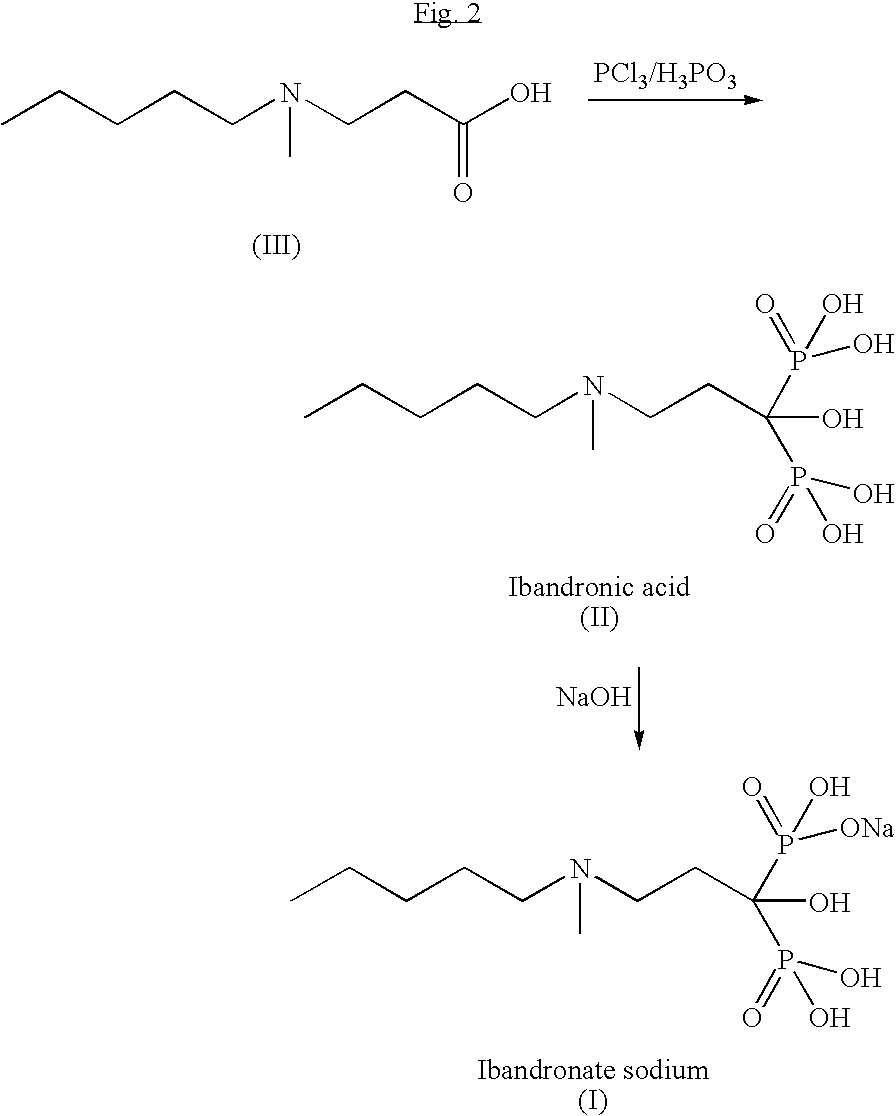
InactiveUS20100228052A1Simple and economical and industrially viable processSpeed up the processOrganic compound preparationSodium organic compoundsPropanoic acidIbandronate Sodium
The present invention relates to an improved process for the synthesis of Ibandronate sodium of formula (I). The present invention also provides novel processes for the synthesis of 3-[N-(methylpentyl)amino]propionic acid (III).
Owner:CIPLA LTD
Antitumor combined medicine
ActiveCN106668067AIncrease lethalityReduce the number of treatmentsOrganic active ingredientsMammal material medical ingredientsTiludronate DisodiumEtidronate Disodium
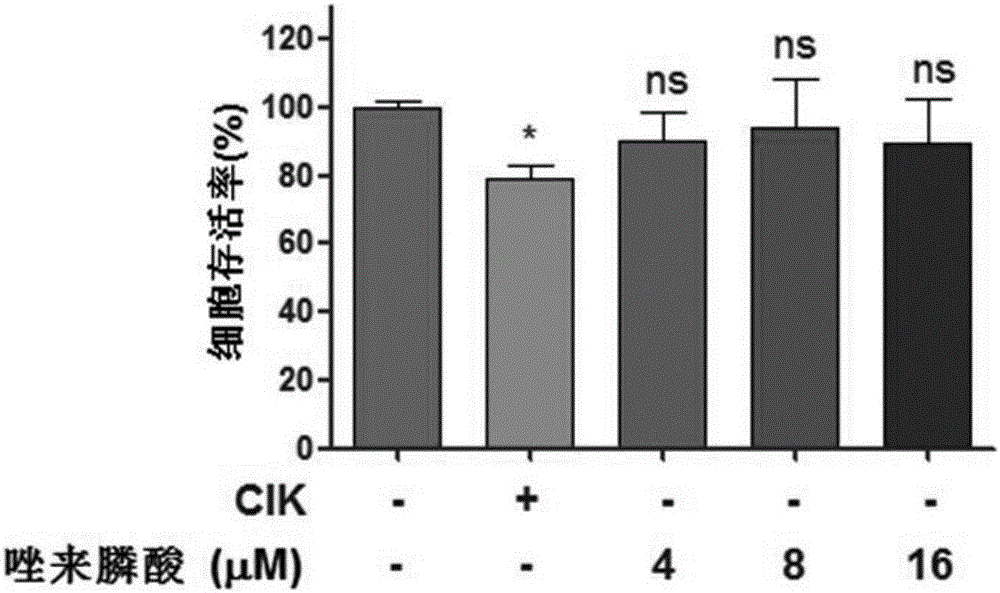
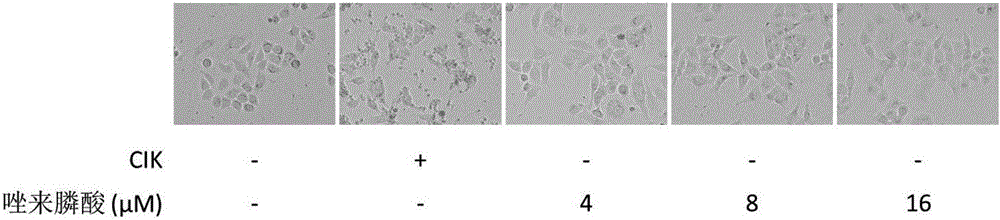
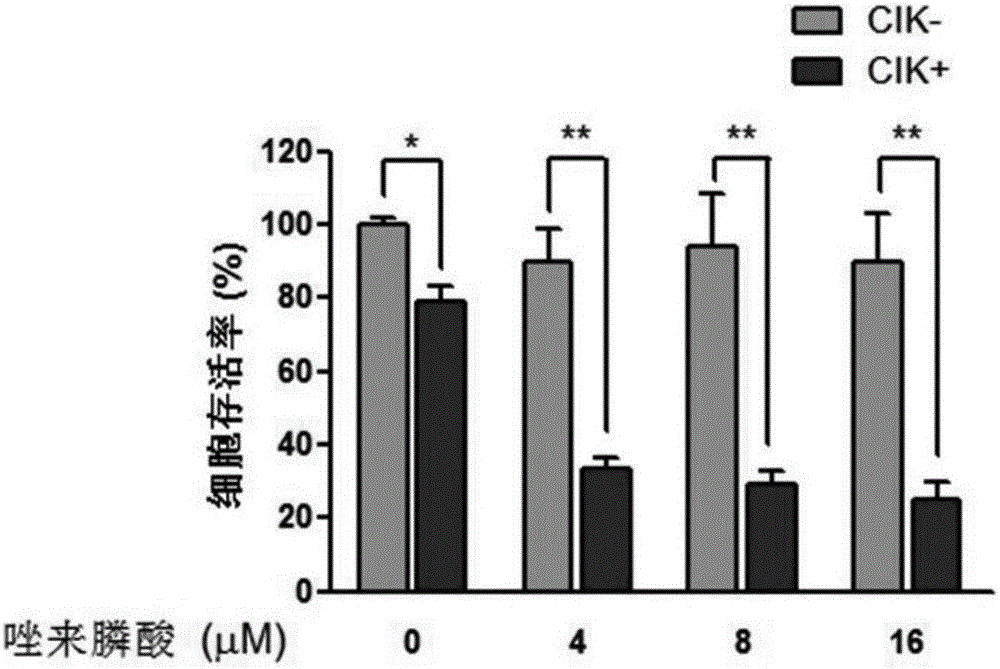
The invention discloses an antitumor combined medicine. The combined medicine comprises a diphosphonate compound serving as an active ingredient and CIK cells, wherein the diphosphonate compound is at least one of clinical medication etidronate disodium, clodronate disodium, pamidronate disodium, tiludronate disodium, alendronate sodium, neridronate sodium, olpadronate sodium, risedronate sodium, sodium ibandronate, incadronate disodium and zoledronic acid. The method for greatly improving the tumor cell killing capability of CIK cells by utilizing combination of anti-tumor-osseous-metastasis diphosphonate medicines and the CIK cells, so that the number of CIK cells for achieving the treatment effect the same as a conventional method is greatly reduced; and compared with biological treatment of single CIK cells, the combined medicine has the advantages that the relatively high safety of CIK cells can be maintained, and the treatment effect can be remarkably improved.
Owner:JINAN UNIVERSITY
Ibandronate sodium containing injection
ActiveCN103070824BStable main drug contentImprove stabilityOrganic active ingredientsInorganic non-active ingredientsActivated carbonPhosphate
The invention relates to an ibandronate sodium containing injection. The injection is prepared by ibandronate sodium and pharmaceutically acceptable auxiliary materials; a pH value of the injection is controlled to be 3.5-4.5 by a phosphate buffer; activated carbon accounting for 0.1% of the total volume of the injection is used for removing a pyrogen; and adsorption of activated carbon to a main medicine is avoided while the pyrogen is removed. An accelerated stability test indicates that the impurity content of the injection is lower than that of the marketed injection, and the quality is stable and controllable.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Preparation method of sodium ibandronate
InactiveCN106397479AReduce the number of refinementsMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsOrganic chemistry methodsPhosphorous acidPropanoic acid
Relating to the technical field of pharmaceutical synthesis, the invention provides a preparation method of sodium ibandronate. The preparation method includes: taking 3-methylamino propanenitrile as the raw material to prepare 3-(N-methyl-N-n-amylamine)propionic acid hydrochloride, then reacting the 3-(N-methyl-N-n-amylamine)propionic acid hydrochloride with phosphorous acid and phosphorus trichloride to obtain ibandronic acid, and subjecting the ibandronic acid and sodium hydroxide to salification so as to obtain sodium ibandronate. The preparation method of sodium ibandronate provided by the invention reduces the refining frequency, lowers the refining loss, avoids the defects of low yield and long period brought about by a lot of refining steps, and the great harm to human and environment, and has the advantages of simple operation, good process repeatability and easy industrial production.
Owner:合肥美利康医药技术股份有限公司
Preparation method of sodium ibandronate
InactiveCN104628768ALow toxicityAvoid reaction stirring difficultiesGroup 5/15 element organic compoundsPhosphorous acidChlorobenzene
The invention relates to a drug synthesis method, and in particular relates to a preparation method of sodium ibandronate. The preparation method comprises the following steps of: directly reacting 3-(N-methyl-N-n-amylamine) propionic hydrochloride, phosphorous acid with phosphorus trichloride to obtain 1-hydroxyl-3-(methyl amylamine)-dimethylmethane-1,1-bipyrophosphoric acid hydrochloride, directly forming salt with sodium hydroxide after hydrolyzing so as to obtain crude sodium ibandronate, and respectively re-crystallizing for one time through mixed solvents of ethanol and water and acetone and water so as to obtain sodium ibandronate. Any solvent is not used in the step of synthesizing pyrophosphoric acid hydrochloride; solid-liquid reaction is carried out without stirring and post-treatment; products are directly used in the next step; a reaction system is dispersed by using organic solvents, such as chlorobenzene and diethyl carbonate, in some reported methods; products are shaped as oil blocks and are not beneficial to stirring and post-treating after reacting; in contrast, the kinds of impurities in the products are reduced; toxicity due to the organic solvents, such as chlorobenzene and diethyl carbonate, is eliminated; furthermore, the stirring difficulty is avoided; sodium ibandronate is prevented from being stuck on a stirrer and a bottle wall; and thus, industrial production is conveniently carried out.
Owner:JIANGSU QINGJIANG PHARMA
Preparation method of ibandronate sodium
InactiveCN103772430AImprove securityLow toxicityGroup 5/15 element organic compoundsSolventIbandronate Sodium
The invention discloses a preparation method of ibandronate sodium, which is characterized in that: adding 3-(N-methyl-N-pentylamino) propionic acid hydrochloride, phosphorous acid, inertia solvent and phosphorus trichloride; carrying out a condensation reaction at 40-80 DEG C for 2-6 hours; adding purified water for 2-6 hours of hydrolysis for generating ibandronate sodium; carrying out a salt forming reaction with sodium hydroxide, and obtaining the ibandronate sodium crude product; recrystallizing the crude product with a mixed solvent containing acetone and water, and obtaining the ibandronate sodium. According to the invention, the added solvent is propylene carbonate, by using the solvent, the reaction resultants can be uniformly dispersed in the solvent, and thereby overcoming the disadvantages that products with viscosity are generated in the reaction by using other solvents, and the products are not easy to be stirred and the reaction is not easy to controlled; and the propylene carbonate has little toxicity; the invention has the advantages of simple operation, high safety, good product quality, low cost, etc. The preparation method is convenient for large scale production.
Owner:LIAONING CHENGDA BIOTECH
Ibandronate sodium propylene glycol solvate and processes for the preparation thereof
InactiveUS20080139845A1Promote resultsImprove purification effectOrganic active ingredientsSkeletal disorderIbandronate SodiumPropylene glycol
A novel form of Ibandronate sodium which is particularly suitable for pharmaceutical applications, and a process for preparing said novel form.
Owner:APOTEX PHARMACHEN INC
Method for preparing 3-(N-methyl-N-pentyl amido) propionate alkoxide
ActiveCN101139298AHigh yieldImprove securityOrganic compound preparationAmino-carboxyl compound preparationDistillationBenzaldehyde
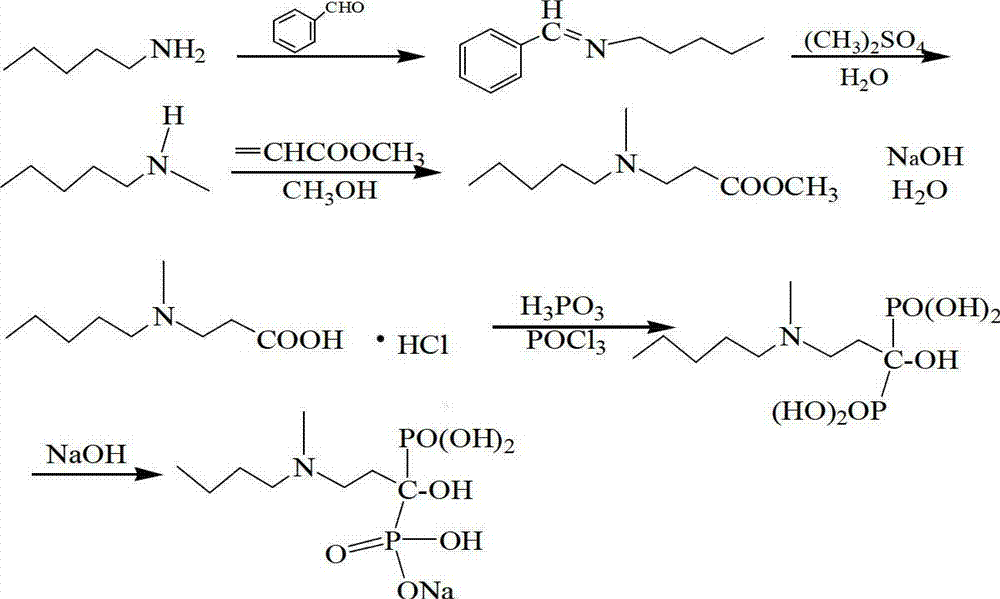
The method discloses a preparation method pf the 3-(N-methyl-N-pentyl amine) propionic hydrochloride, aiming to provide a preparation method of the e 3-(N-methyl-N-pentyl amine) propionic hydrochloride with the high collection rate, the simple operation, the difficulty of moisture absorption, the high security, the small loss and other advantages; the method is suitable for the large-scale industrial production. In the method, the pentyl amine, benzaldehyde, dimethyl sulfate and alkali are used as the raw materials; the N-methyl pentyl amine is synthesized after the dehydration, addition and distillation; the N-methyl pentyl amine, 3-halogenated propionate and alkali are used as the raw materials; the 3-(N-methyl-N-pentyl amine) propionic ester is synthesized in the solvent through the Hockman Addition; the 3-(N-methyl-N-pentyl amine) propionic ester is then used as the raw material; and the 3-(N-methyl-N-pentyl amine) propionic hydrochloride can be got after the hydrolysis and acidification. The product of the present invention can be used as the drug intermediate for the synthesis of the Iban sodium phosphonic.
Owner:CHENGDA PHARM CO LTD
Medicine compounds for treating osteoporosis
The invention provides a medical compound for treating osteoporosis which is characterized in that the invention contains strontium ranelate and bisphosphonate. Animal experiments indicate that the invention achieves the unexpected effect for treating the osteoporosis. The osteoporosis is a bone disease of the whole body characterized by the low bone mass and the degeneration of the micro structure of the bone organization, companying with the enhancement of the bone fragility and easy happened bone broken for which no ideal treatment medicine exists in the clinic. The bisphosphonate of the invention comprises alendronate, risedronate sodium, ibandronate, pamidronate, Etidronate, disodium clodronate and zoledronic acid, etc. In the invention, the dosage of the bisphosphonate is greatly reduced, which can effectively reduce the happening of side effects and is convenient to use the medicine.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing sodium ibandronate crystal and hydrated crystal thereof
InactiveCN101117339AComposition is stableAppropriate bulk densityGroup 5/15 element organic compoundsOrganic solventGlycol ethers
The invention provides a preparation process for Ibandronate monosodium crystals and hydration crystals thereof. The Ibandronate monosodium compound in Formula (1) is dissolved in 2 to 30 times of water; at least one of azanyl amides, alkyl sulfoxides, glycol ethers and alicyclic ethers is added into the compound solution, is stirred under condition of room-temperature to precipitate crystals, then is cooled down to minus 5 to 15 DEG C and filtered, and is dried in vacuum between 30 and 75 DEG C. The process is simple and easy to operate; Ibandronate monosodium hydration crystals have good crystal stability and are suitable for industrial production and storage.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Sodium ibandronate injection composition and preparation method of sodium ibandronate injection composition
ActiveCN103239396BSimple prescriptionReduce contentOrganic active ingredientsMetabolism disorderAcetic acidSodium acetate
The invention provides a sodium ibandronate injection composition. Every 1000 injections consist of the following components: 1.0684-6.4101g of sodium ibandronate, 1.66-9.96g of sodium acetate, suitable amount of glacial acetic acid, and 1000-6000ml of water for injection. The sodium ibandronate injection prepared by the invention has the advantages that the prescription is simple, auxiliary materials are all commonly used by the injection, and the safety is high. Simultaneously the sodium acetate content is low, irritation cannot be produced in intravenous administration, the prepared final product is stable in quality, and the indexes of related substances and contents are all superior to those in the prior art.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Preparation method of sodium ibandronate
InactiveCN102898466BEasy to operateMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidChlorobenzene
The invention relates to the technical field of pharmaceutical chemistry, particularly relates to a method of pharmaceutical synthesis, and specifically relates to a preparation method of sodium ibandronate. To overcome the disadvantages of high content of chlorides and phosphites in sodium ibandronate prepared by a conventional preparation method of sodium ibandronate, the preparation method of sodium ibandronate with extremely low content of chlorides and phosphites is provided. In the preparation method, 3-(N-methylpentylamino) propionic acid hydrochloride, phosphorus trichloride and phosphorous acid are employed as raw materials and reacted in a chlorobenzene solvent, so as to obtain sodium ibandronate with extremely low content of the chlorides and the phosphites. The obtained sodium ibandronate can not only meet impurity control standards of the chlorides and the phosphites in a sodium ibandronate crude drug, but also prevent low yield, long period and huge harm to human body and environment which are brought by a lot of refining steps.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing sodium Ibandronate monohydrate
ActiveCN103554177AIn line with clinical medicineLow toxicityGroup 5/15 element organic compoundsPhosphorous acidChemical synthesis
The invention belongs to the field of medicine chemistry synthesis, and relates to a method for preparing a sodium Ibandronate monohydrate. The method comprises that: 1) 3-(N-methylpentylamino)propanoic acid hydrochloride, phosphorous acid and phosphorus trichloride are adopted as starting raw materials and are subjected to a reaction in a 1,3-dimethyl-2-imidazolidinone (DMI) solvent or a DMI-containing mixing solvent to obtain the sodium Ibandronate monohydrate crude product meeting the internal control standard in the one-step manner; and 2) the sodium Ibandronate monohydrate crude product is subjected to recrystallizatio in a methanol / water mixing solvent to obtain the sodium Ibandronate monohydrate meeting the clinical medicinal standard. According to the present invention, defects of low yield, high reagent toxicity, heavy pollution and the like in the prior art are overcome, and the preparation method for the sodium Ibandronate monohydrate represented by the formula (I) is provided, wherein the preparation method has characteristics of high yield, low environmental pollution, and easy purification.
Owner:ANHUI PIOM PHARMA
Ibandronate sodium propylene glycol solvate and processes for the preparation thereof
InactiveUS7696183B2Improve purification effectRobust and cost-effectiveBiocideGroup 5/15 element organic compoundsIbandronate SodiumPropylene glycol
A novel form of Ibandronate sodium which is particularly suitable for pharmaceutical applications, and a process for preparing said novel form.
Owner:APOTEX PHARMACHEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com