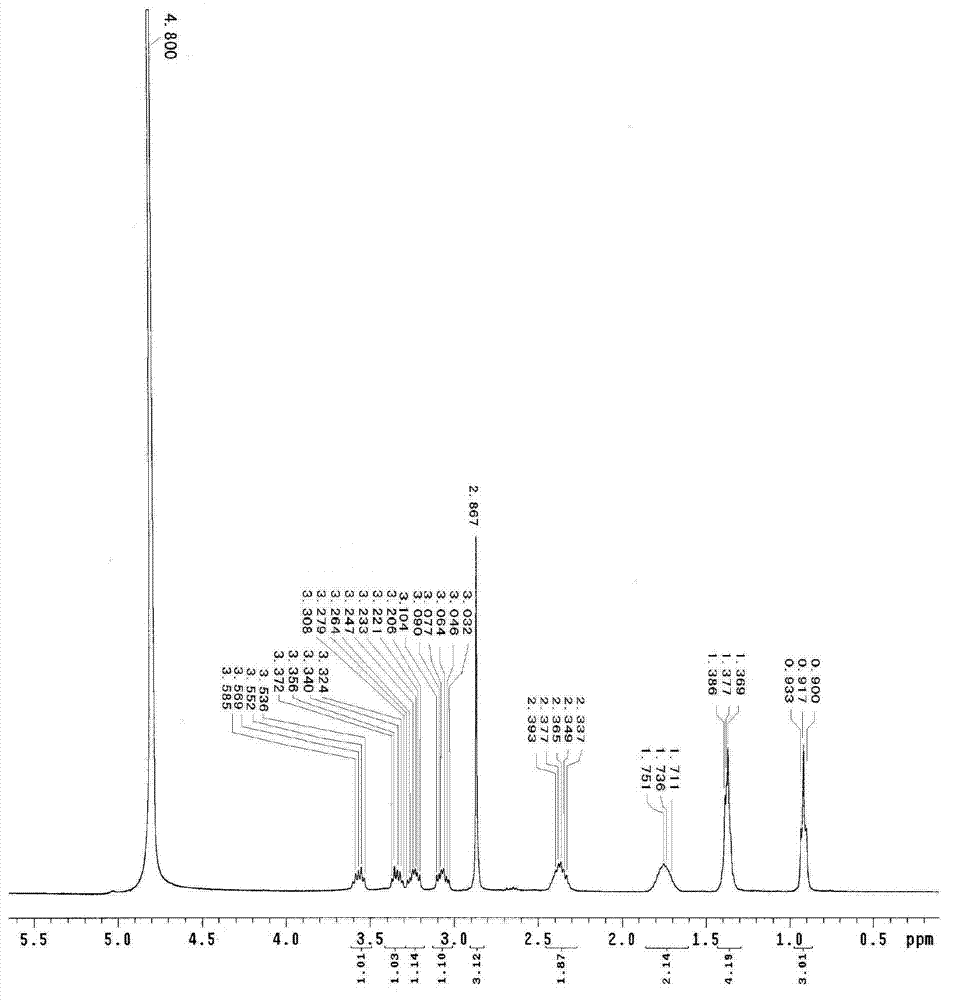
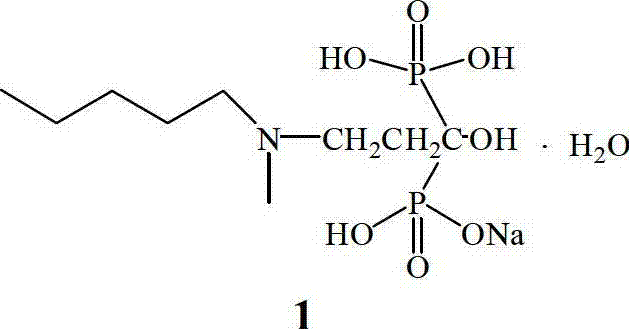
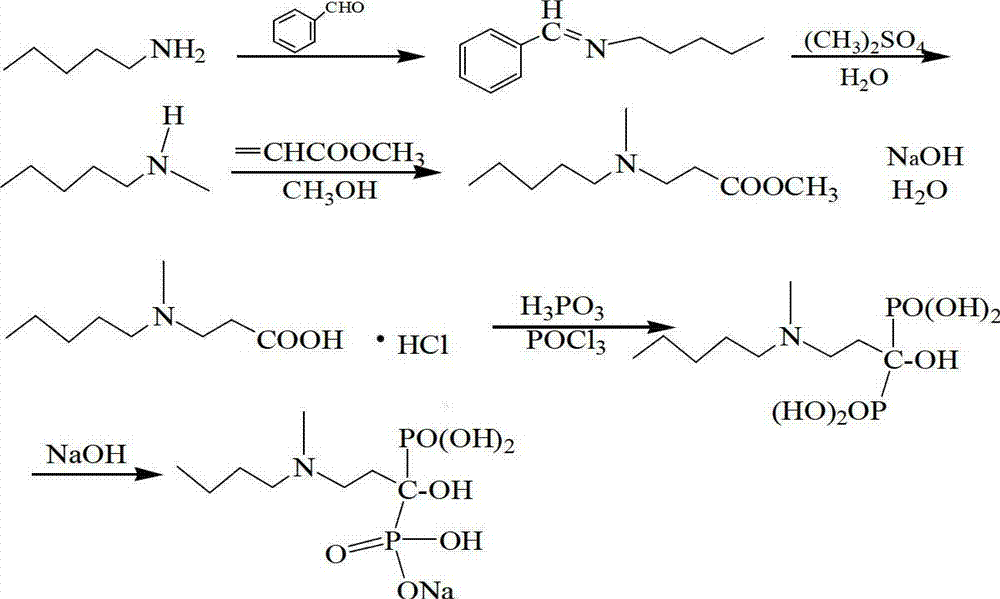
Method for preparing sodium ibandronate
The technology of sodium ibandronate and ibandronic acid is applied in the field of preparation of sodium ibandronate, can solve the problems of low yield of sodium ibandronate, high production cost, many steps and the like, and achieves high purity, The effect of few reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] One aspect of the present invention provides a kind of preparation method of sodium ibandronate, comprising the following steps:
[0035] 1) Bromination reaction of 3-methylaminopropionitrile with n-bromopentane in N,N-dimethylformamide solution of anhydrous potassium carbonate to obtain 3-(N-methyl-N-pentyl)amino propionitrile;
[0036] 2) Hydrolyzing 3-(N-methyl-N-pentyl)aminopropionitrile and a phase transfer catalyst under alkaline conditions to obtain 3-(N-methyl-N-pentyl)aminopropionic acid;
[0037] 3) Diphosphonate and hydrolyze 3-(N-methyl-N-pentyl)alanine, phosphorous acid, and thionyl chloride in the presence of toluene to obtain ibandronic acid;
[0038] 4) neutralizing ibandronic acid under alkaline conditions to obtain crude ibandronic acid sodium;
[0039] 5) The crude product of sodium ibandronate is subjected to a crystallization and purification step in an aqueous organic solvent to obtain sodium ibandronate.
[0040] The structural formula of above...
Embodiment 1
[0063] 1) Add 47.2g (0.562mol) of 3-methylaminopropionitrile and 150mL of N,N-dimethylformamide into a 500ml dry three-necked flask, stir well at room temperature, add 50g (0.362mol) of anhydrous potassium carbonate, Add 94.8g (0.628mol) of n-bromopentane dropwise, heat up to 40°C and stir for 2h, then add 50g (0.362mol) of anhydrous potassium carbonate, stir mechanically at 80°C for 4h, then quench with 200mL of ice water Reaction, then stirred for 1h to obtain 3-(N-methyl-N-pentyl)aminopropionitrile liquid, 3-(N-methyl-N-pentyl)aminopropionitrile liquid was extracted 3 times with 150ml of toluene respectively The organic layer was obtained, the organic layer was separated, and then washed twice with 300ml of saturated brine to obtain the organic phase, the organic phase was dried with anhydrous sodium sulfate for 2h, then filtered to obtain the filtrate, and the filtrate was concentrated under reduced pressure to obtain 85.3 g of yellow liquid 3-(N-methyl-N-pentyl)aminopropi...
Embodiment 2
[0069]1) Add 47.2g (0.562mol) of 3-methylaminopropionitrile and 150mL N,N-dimethylformamide into a 500ml dry three-necked flask, stir well at room temperature, add 100g (0.724mol) of anhydrous potassium carbonate, drop Add 94.8g (0.628mol) of n-bromopentane, heat up to 40°C and stir for 2h, then heat up to 80°C for 4h, then quench the reaction with 200mL of ice water, and stir for 1h to obtain 3-(N- Methyl-N-pentyl)aminopropionitrile liquid and 3-(N-methyl-N-pentyl)aminopropionitrile liquid were extracted three times with 150ml of toluene solution to obtain an organic layer. The organic layer was washed twice with 300 ml of saturated brine to obtain an organic phase. The organic phase was dried with anhydrous sodium sulfate for 2 h, and then filtered to obtain a filtrate. The filtrate was concentrated under reduced pressure to obtain 82.5 g of light yellow 3-(N-methyl-N-pentyl)aminopropionitrile liquid with a slightly pungent smell.
[0070] 2) In a 500ml three-necked flask,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com