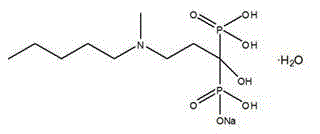
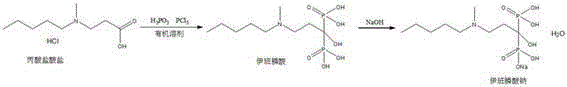
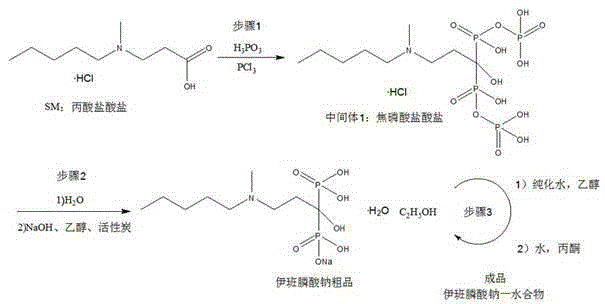
Preparation method of sodium ibandronate
A technique for sodium ibandronate and a synthetic method, which is applied in the field of drug synthesis technology, can solve problems such as low yield, many types of impurities of sodium ibandronate, and difficult reaction and stirring, so as to achieve good repeatability and avoid difficult reaction and stirring , the effect of facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Synthesis of pyrophosphate hydrochloride
[0034] Add 5.5kg of propionate hydrochloride and 5.2kg of phosphorous acid into the reaction kettle, stir and heat to 75-80°C, the solid melts and becomes transparent, stir vigorously, add 5.55L of phosphorus trichloride dropwise at 70-80°C for 1-2 hours, And a large amount of acid gas is released; after the dropwise addition, the solution solidifies slowly, and the stirring is stopped for 6 hours; TLC monitors, and the reaction is cooled to room temperature after the completion of the reaction. Proceed directly to the next step.
[0035] 2. Synthesis of Crude Sodium Ibandronate
[0036] Add 20L of purified water to the reaction kettle containing pyrophosphate hydrochloride, slowly raise the temperature to reflux; after reflux for 6-10 hours, cool down to room temperature, add dropwise 50% sodium hydroxide solution to pH = 4.1-4.8 (about 5L ), add activated carbon and stir at room temperature for 0.5 hours, and filter. Tr...
Embodiment 2
[0041] 1. Synthesis of pyrophosphate hydrochloride
[0042] Add 5.5kg of propionate hydrochloride and 5.2kg of phosphorous acid into the reaction kettle, stir and heat to 75-80°C, the solid melts and becomes transparent, stir vigorously, add 5.55L of phosphorus trichloride dropwise at 70-80°C for 1-2 hours, And a large amount of acid gas is released; after the dropwise addition, the solution solidifies slowly, and the stirring is stopped for 6 hours; TLC monitors, and the reaction is cooled to room temperature after the completion of the reaction. Proceed directly to the next step.
[0043] 2. Synthesis of Crude Sodium Ibandronate
[0044] Add 20L of purified water to the reaction kettle containing pyrophosphate hydrochloride, slowly raise the temperature to reflux; after reflux for 6-10 hours, cool down to room temperature, add dropwise 50% sodium hydroxide solution to pH = 4.1-4.8 (about 5L ), add activated carbon and stir at room temperature for 0.5 hours, and filter. Tr...
Embodiment 3
[0049] 1. Synthesis of pyrophosphate hydrochloride
[0050] Add 5.5kg of propionate hydrochloride and 5.2kg of phosphorous acid into the reaction kettle, stir and heat to 75-80°C, the solid melts and becomes transparent, stir vigorously, add 5.55L of phosphorus trichloride dropwise at 70-80°C for 1-2 hours, And a large amount of acid gas is released; after the dropwise addition, the solution solidifies slowly, and the stirring is stopped for 6 hours; TLC monitors, and the reaction is cooled to room temperature after the completion of the reaction. Go directly to the next step.
[0051] 2. Synthesis of Crude Sodium Ibandronate
[0052] Add 20L of purified water to the reaction kettle containing pyrophosphate hydrochloride, slowly raise the temperature to reflux; after reflux for 6-10 hours, cool down to room temperature, add dropwise 50% sodium hydroxide solution to pH = 4.1-4.8 (about 5L ), add activated carbon and stir at room temperature for 0.5 hours, and filter. Transfe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com