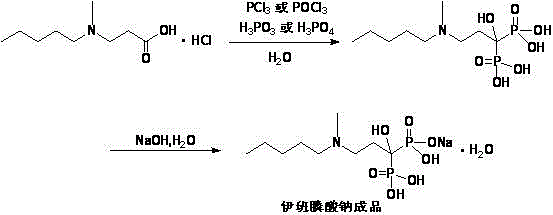
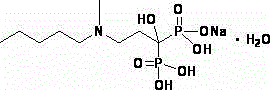
Preparation method of sodium ibandronate
A technology of sodium ibandronate and propionate hydrochloride, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, blood diseases, etc., can solve the problem of low chloride and phosphite content , high chloride and phosphite content, etc., to achieve the effect of easy industrial production, high product purity and long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 comparative test
[0026] The preparation of sodium ibandronate of the present invention
[0027] Add 10.5g (0.050mol) of 3-(N-methyl-n-pentylamino) propionate hydrochloride, 10.3g (0.125mol) of phosphorous acid and 50ml of chlorobenzene into the reaction flask, stir and heat to 80°C, add three Phosphorus chloride 11ml (0.125mol), after dripping, keep warm for 9 hours, discard the supernatant chlorobenzene solution, add 0.1mol / L hydrochloric acid solution 160ml, heat to 90°C and keep warm for 10 hours, then filter, and concentrate the filtrate under reduced pressure , 42ml of purified water to dissolve the residue, add petroleum ether (60-90℃) to extract twice, 21ml each time, discard the petroleum ether layer, collect the water layer, concentrate to obtain the residue ibandronic acid, add 42ml of purified water to dissolve and use Adjust the pH to 4.3 with 30% sodium hydroxide solution, add 42 ml of tetrahydrofuran dropwise, crystallize, and filter to ...
Embodiment 2
[0035] Example 2 Stability test
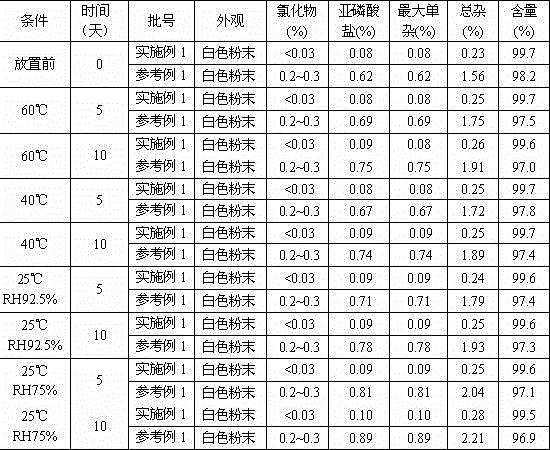
[0036] Take the samples of Example 1 (batch number: 100601 batches) and reference example 1 (100102 batches), put them in a petri dish, spread them into a thin layer ≤ 5mm thick, and place them in high temperature (60°C, 40°C), high humidity (25 ℃ RH92.5%, 25℃ RH75%±5%) for 10 days, samples were taken on the 5th and 10th day. The results are shown in Table 1 below.
[0037] Table 1 The results of the experimental investigation on the influencing factors of ibandronate sodium
[0038]
[0039]Conclusion: The samples of Example 1 and Reference Example 1 were placed at high temperature 60°C, 40°C and high humidity RH75%, 92.5% for 5 and 10 days respectively. The appearance, chloride, There was no significant change in the indicators of phosphite, maximum single impurity and total impurity of related substances, content, etc.; chloride, phosphite, maximum single impurity and total impurity of related substances were higher in the samples of...
Embodiment 3
[0040] Example 3 Preparation of Sodium Ibandronate
[0041] Add 21g (0.10mol) of 3-(N-methyl-n-pentylamino) propionate hydrochloride, 20.5g (0.25mol) of phosphorous acid and 210ml of chlorobenzene into the reaction flask, stir and heat to 90°C, and drop trichloro Phosphate 22ml (0.25mol), after dripping, keep warm for 12 hours, discard the supernatant chlorobenzene solution, add 0.1mol / L hydrochloric acid solution 420ml, heat to 80°C and keep warm for 12 hours, filter, evaporate the solvent under reduced pressure , 84ml of purified water to dissolve the residue, add petroleum ether (30-60°C) to extract twice 84ml each time, collect the water phase and concentrate to obtain the residue, add 84ml of purified water to dissolve, adjust the pH to 4.0 with 30% sodium hydroxide solution, 84 ml of tetrahydrofuran was added dropwise, crystallized, and 26.3 g of sodium ibandronate was obtained by filtration, with a yield of 73.2%.
[0042] Volumetric titration test: content 99.5%; HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com