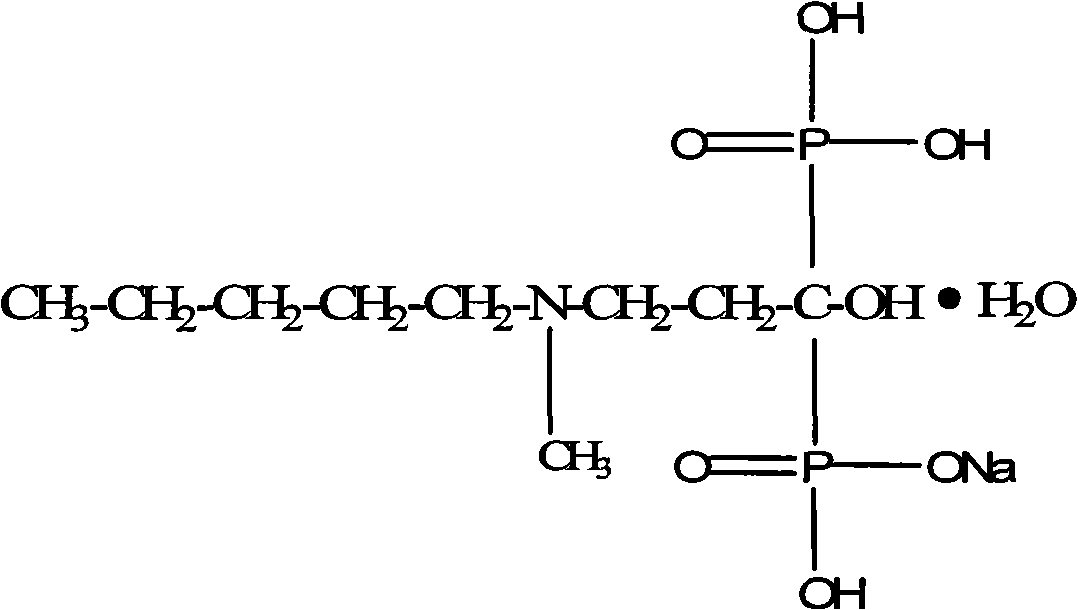
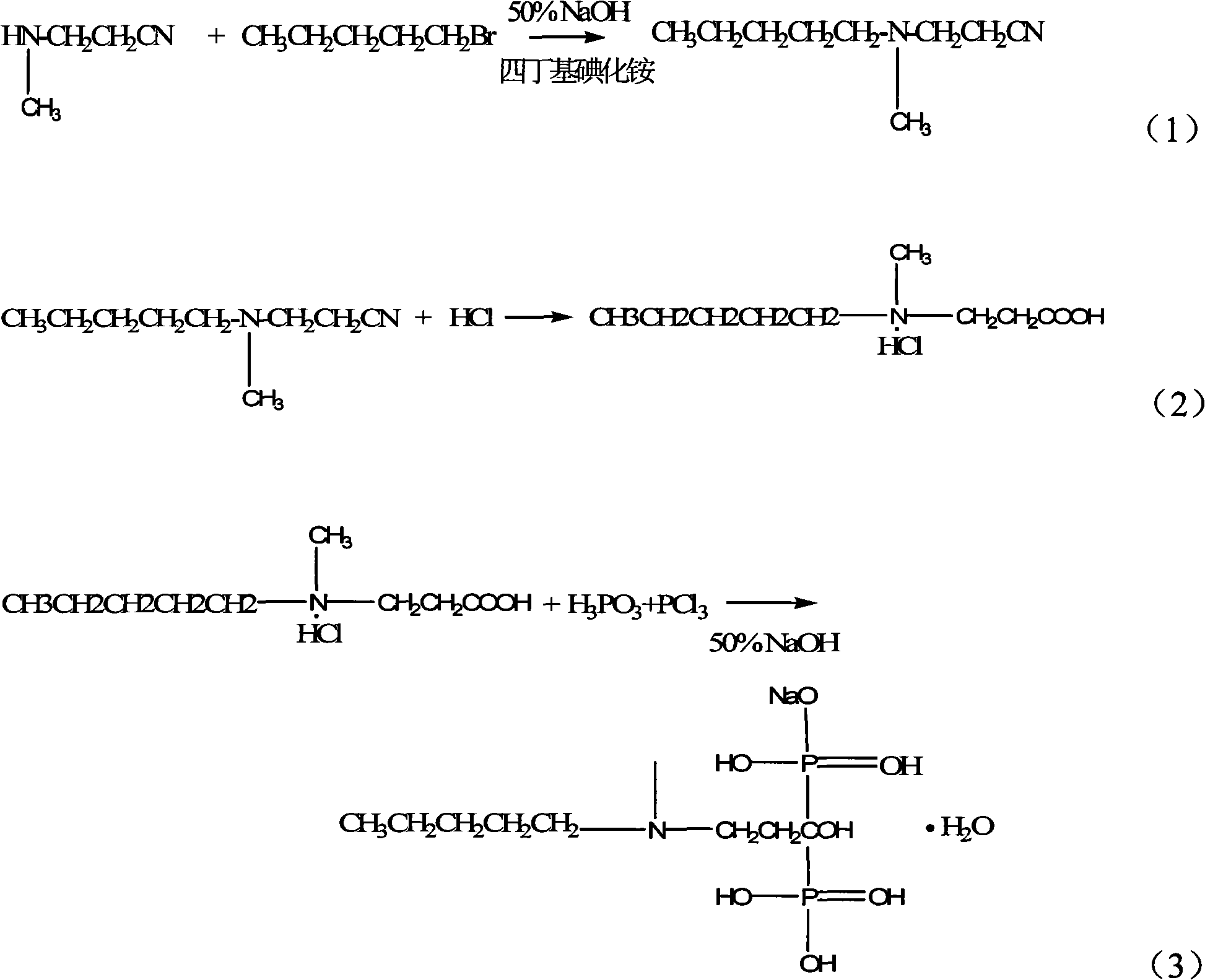
Synthetic method of ibandronate
A technology of sodium ibandronate and synthesis method, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, drug combinations, etc., can solve problems such as low total yield and low yield, and achieve The effect of low production cost, high product yield and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, step one:
[0031] Raw material name Mass (g) Molecular mass Molar mass Molar ratio
[0032] 3-Methylaminopropionitrile (>98%) 85.8 84.1 1.0 1.0
[0033] 1-Bromopentane (>98%) 169.5 151 1.1 1.1
[0034] NaOH (>96%) 50.0 40 1.2 1.2
[0035] Tetrabutylammonium iodide (>99%) 3.7 369 0.01 0.01
[0036] Water 50.0 18 2.67 2.67
[0037] Toluene 216.5 (250ml) 92.14 2.35 2.35
[0038] 1. Add NaOH aqueous solution, 50ml (43.3g) toluene, tetrabutylammonium iodide, and 3-methylaminopropionitrile into a 500ml three-neck flask, install a general-purpose mercury thermometer, and stir mechanically, which is a common mechanical stirrer in the laboratory , water bath, stir for half an hour, and mix well;
[0039] 2. Maintain the temperature at 45°C and add 1-bromopentane dropwise, use a dropping funnel for two hours, stir at 45°C for 7 hours, use Shimadzu gas chromatography, model GC-14C, monitor until the end of the reaction;
[0040] 3. Add 200ml of water to disso...
Embodiment 2
[0077] Embodiment 2, step one:
[0078] Raw material name Mass (g) Molecular mass Molar mass Molar ratio
[0079] 3-Methylaminopropionitrile (>98%) 84.1 84.1 0.98 1.0
[0080] 1-Bromopentane (>98%) 160.0 151 1.04 1.06
[0081] NaOH (>96%) 44.0 40 1.06 1.08
[0082] Tetrabutylammonium iodide (>99%) 7.4 369 0.02 0.02
[0083] Water 44.0 18 2.67 2.72
[0084] Toluene 217 (250ml) 92.14 1.88 1.92
[0085]1. Add NaOH aqueous solution, 50ml toluene, tetrabutylamine iodide, and 3-methylaminopropionitrile into a three-necked flask, install a thermometer, stir mechanically, and stir in a water bath for half an hour, and mix well;
[0086] 2. Maintain the temperature at 25°C and add 1-bromopentane dropwise for two hours, stir at 25°C for 7 hours, and monitor by gas chromatography until the reaction is complete;
[0087] 3. Add 200ml of water to dissolve the solid and separate the liquid;
[0088] 4. The lower layer was extracted twice with 100ml×2 toluene, the organic phases were ...
Embodiment 3
[0124] Embodiment 3, step one:
[0125] Raw material name Mass (g) Molecular mass Molar mass Molar ratio
[0126] 3-Methylaminopropionitrile (>98%) 42.1 84.1 0.50 1
[0127] Pentyl bromide (>98%) 80.0 151 0.53 1.06
[0128] NaOH (>96%) 20.8 40 0.5 1
[0129] Tetrabutylammonium iodide (>99%) 3.7 369 0.01 0.02
[0130] Water 18.0 18 1.0 2
[0131] Toluene 104 (120ml) 92.14
[0132] 1. Add 50% NaOH aqueous solution, 20ml toluene, tetrabutylammonium iodide, and 3-methylaminopropionitrile into a 250ml three-necked flask, install a general-purpose mercury thermometer, magnetically stir, and stir in a 25-degree water bath for half an hour, and mix well.
[0133] 2. Add bromide pentane dropwise at 25°C, finish dropping in two hours, stir for 15 hours, and monitor by gas chromatography until the peak of the raw material disappears.
[0134] 3. Add 100ml of water to dissolve the solid and separate the liquid.
[0135] 4. The lower layer was extracted with 100ml of toluene, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com