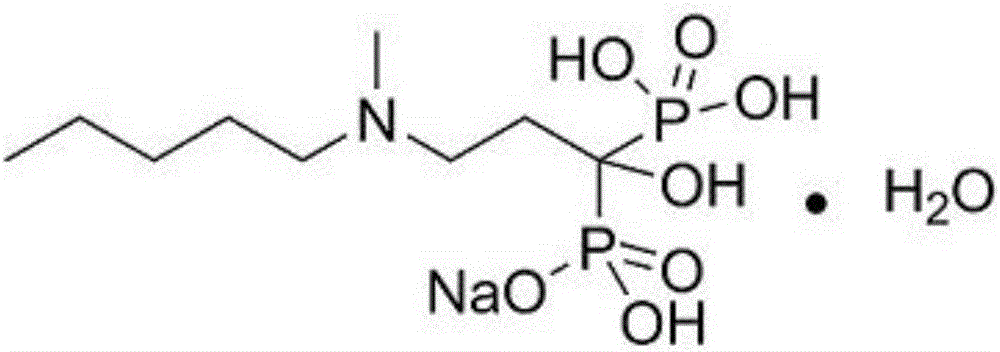
Preparation method of sodium ibandronate
A technology of sodium ibandronate and propionate hydrochloride is applied in the field of preparation of sodium ibandronate, which can solve the problems of affecting the continuation of the reaction, difficult and large-scale production of the process, low product yield and the like, and simplify the production process. , Reducing the number of refining times, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method of ibandronate sodium, comprising the following steps:
[0021] (1) Add 3-methylaminopropionitrile, alkali metal solution and phase transfer catalyst into N,N-dimethylformamide, mix well, add 1-bromopentane dropwise, stir at room temperature 40°C for 3h, and obtain 3 -(N-methyl-N-n-pentylamine) propionitrile liquid crude product;
[0022] (2) 3-(N-Methyl-N-n-pentylamine) propionitrile liquid crude product is mixed with hydrochloric acid in a weight ratio of 1:4, heated to 90°C for reflux reaction for 24 hours, the hydrochloric acid is removed by vacuum distillation, and toluene is azeotroped Obtain 3-(N-methyl-N-n-pentylamine) propionate hydrochloride after distilling off water;
[0023] (3) Add 3-(N-methyl-N-pentylamino) propionate hydrochloride and phosphorous acid in acetonitrile, wherein 3-(N-methyl-N-n-pentylamine) propionate hydrochloride The molar ratio of salt to phosphorus trichloride is 1:1.2. After stirring evenly, add tetrabutylammoniu...
Embodiment 2
[0025] A preparation method of ibandronate sodium, comprising the following steps:
[0026] (1) Add 3-methylaminopropionitrile, alkali metal solution and phase transfer catalyst to propylene carbonate, mix well, add 1-bromopentane dropwise, and stir at room temperature for 2 hours at 50°C to obtain 3-(N-methyl -N-n-pentylamine) propionitrile liquid crude product;
[0027] (2) 3-(N-Methyl-N-n-pentylamine) propionitrile liquid crude product is mixed with hydrochloric acid at a weight ratio of 1:5, heated to 120°C for reflux reaction for 4 hours, distilled under reduced pressure to remove hydrochloric acid, and azeotroped toluene Obtain 3-(N-methyl-N-n-pentylamine) propionate hydrochloride after distilling off water;
[0028] (3) Add 3-(N-methyl-N-pentylamino) propionate hydrochloride and phosphorous acid in acetonitrile, wherein 3-(N-methyl-N-n-pentylamine) propionate hydrochloride The molar ratio of salt to phosphorus trichloride is 1:1.5. After stirring evenly, add benzyltri...
Embodiment 3
[0030] A preparation method of ibandronate sodium, comprising the following steps:
[0031] (1) Add 3-methylaminopropionitrile, alkali metal solution and phase transfer catalyst to isopropanol, mix well, add 1-bromopentane dropwise, stir at room temperature 45°C for 2h, and obtain 3-(N-methyl -N-n-pentylamine) propionitrile liquid crude product;
[0032] (2) 3-(N-Methyl-N-n-pentylamine) propionitrile liquid crude product is mixed with hydrochloric acid in a weight ratio of 1:8, heated to 110°C for reflux reaction for 8 hours, the hydrochloric acid is removed by vacuum distillation, and toluene is azeotroped Obtain 3-(N-methyl-N-n-pentylamine) propionate hydrochloride after distilling off water;
[0033] (3) Add 3-(N-methyl-N-pentylamino) propionate hydrochloride and phosphorous acid in acetonitrile, wherein 3-(N-methyl-N-n-pentylamine) propionate hydrochloride The molar ratio of salt to phosphorus trichloride is 1:1.5. After stirring evenly, add hexadecyltributylammonium bro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com