Resolution method of N-methyl-2(2-hydroxyethyl)pyrrolidine and application thereof
A technology of hydroxyethyl and pyrrolidine, applied in the field of drug synthesis, can solve problems affecting the optical purity of the final product, racemization of raw materials, and easy racemization, etc., to improve the optical purity of the product, not easy to racemize, Ease of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
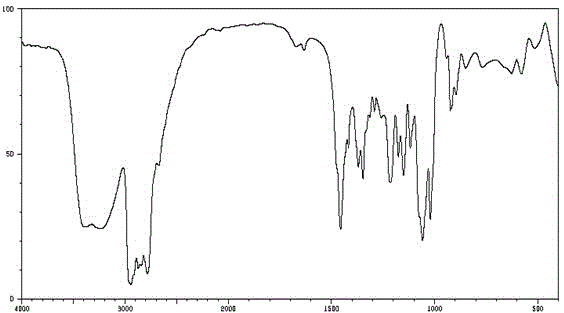
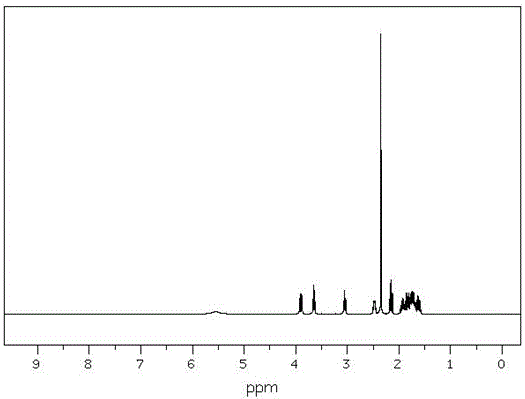
Image
Examples
Embodiment 1
[0047] (1) Put 37.6 grams of L-(-)-dibenzoyl tartaric acid monohydrate into 14.84 grams of ethanol, heat to dissolve, and obtain solution Ⅰ
[0048] (2) Put 12.9 grams of N-methyl-2-(2-hydroxyethyl)pyrrolidine racemate into 90.3 grams of ether, stir and mix evenly to obtain solution II
[0049] (3) Slowly pour solution II into slowly stirred solution I to obtain cloudy solution III.
[0050] (4) Place the cloudy solution III at room temperature for crystallization for 1 hour; then put the cloudy solution in an ice-water bath at 0~-5°C and let it stand for crystallization for 4 hours.
[0051] (5) Filter, wash the filter cake with a mixture of ethanol and ether, and drain.
[0052] (6) Put the filter cake into 200ml of water, cool down in an ice bath, adjust the pH value to 13 with sodium hydroxide at 0~5°C, extract with chloroform three times, each time with 80ml of chloroform.
[0053] (7) The chloroform layers were combined and dried by adding 50 g of anhydrous sodium sulf...
Embodiment 2
[0061] (1) Put 45.2 grams of L-(-)-dibenzoyl tartaric acid monohydrate into 15.82 grams of methanol, heat and dissolve to obtain solution Ⅰ
[0062] (2) Put 12.9 grams of N-methyl-2-(2-hydroxyethyl)pyrrolidine racemate into 90.3 grams of ether, stir and mix evenly to obtain solution II
[0063] (3) Slowly pour solution II into slowly stirred solution I to obtain a cloudy solution.
[0064] (4) The turbid solution was crystallized at room temperature for 3 hours. Then put the turbid solution into an ice-water bath at 0~-5°C, and let it crystallize for 8 hours.
[0065] (5) Filter, wash the filter cake with a mixture of methanol and ether, and drain.
[0066] (6) Put the filter cake into 200ml of water, cool down in an ice bath, adjust the pH value to 13 with potassium hydroxide solution at 0~5°C, and extract with dichloromethane 3 times, each time with 80ml of dichloromethane.
[0067] (7) The dichloromethane layers were combined and dried by adding 50 g of anhydrous magnesi...
Embodiment 3
[0071] (1) Put 41.4 grams of L-(-)-dibenzoyl tartaric acid monohydrate into 22.8 grams of ethanol, heat and dissolve to obtain solution Ⅰ
[0072] (2) Put 12.9 grams of N-methyl-2-(2-hydroxyethyl)pyrrolidine racemate into 129 grams of methyl tert-butyl ether, stir and mix evenly to obtain solution II
[0073] (3) Slowly pour solution II into slowly stirred solution I to obtain a cloudy solution.
[0074] (4) The turbid solution was crystallized at room temperature for 3 hours. Then put the turbid solution into an ice-water bath at 0~-5°C, and let it crystallize for 8 hours.
[0075] (5) Filter, wash the filter cake with a mixture of ethanol and methyl tert-butyl ether, and drain it.
[0076] (6) Put the filter cake into 200ml of water, cool down in an ice bath, adjust the pH value to greater than 13 with sodium hydroxide solution at 0-5°C, and extract with dichloroethane 3 times, each time with 80ml of dichloroethane.
[0077] (7) The dichloroethane layers were combined and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com