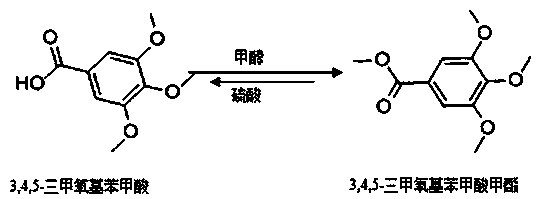
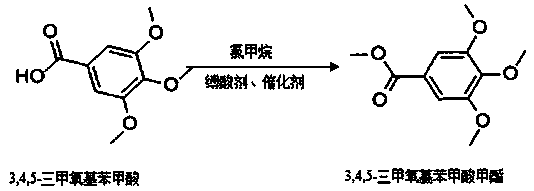
A kind of synthetic method of methyl 3,4,5-trimethoxybenzoate
A technology of methyl trimethoxybenzoate and trimethoxybenzoic acid, which is applied in the field of chemistry, can solve problems such as incomplete reaction, achieve the effects of complete reaction of raw materials, reduce production costs, and reduce waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: a kind of synthetic method of 3,4,5-trimethoxymethylbenzoate, concrete method and step are:
[0026] (1) Add 20g of 3,4,5-trimethoxybenzoic acid, 80ml of DMF, 4g of sodium hydroxide, and 2g of sodium bromide into a three-necked flask, stir, cool down to below 10°C, and inject 6g of methyl chloride gas. After passing through, the temperature was raised to about 45°C, and the reaction was kept for 5 hours;
[0027] (2) After the reaction is completed, distill and concentrate, add the concentrated solution to 200ml of experimental water, stir and cool down to room temperature, let stand, precipitate and then suction filter to obtain the crude product;
[0028] (3) Put the crude product into 80ml of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After concentrated distillation, suction filtration and drying, 18.2g of pure product was obtained, the yield was about 85.4%, the content was 99.89% by HPLC, and the ...
Embodiment 2
[0029] Embodiment 2: a kind of synthetic method of 3,4,5-trimethoxymethylbenzoate, concrete method and step are:
[0030] (1) Add 20g of 3,4,5-trimethoxybenzoic acid, 150ml of DMF, 5g of sodium carbonate, and 3g of sodium bromide into a three-neck flask, stir, cool down to below 10°C, and inject 6g of methyl chloride gas. After passing through, the temperature was raised to about 50°C, and the heat preservation reaction was carried out for 4 hours;
[0031] (2) After the reaction is completed, distill and concentrate, add the concentrated solution to 300ml of experimental water, stir and cool down to room temperature, let stand, precipitate and then suction filter to obtain the crude product;
[0032] (3) Put the crude product into 60ml of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After concentrated distillation, suction filtration and drying, 18.0 g of pure product was obtained, the yield was about 84.5%, the content was...
Embodiment 3
[0033] Embodiment 3: a kind of synthetic method of 3,4,5-trimethoxymethylbenzoate, concrete method and step are:
[0034] (1) Add 60g of 3,4,5-trimethoxybenzoic acid, 300ml of DMF, 18g of sodium carbonate, and 12g of sodium bromide into a three-necked flask, stir, cool down to below 10°C, and inject 18g of methyl chloride gas. After passing through, the temperature is raised to about 55°C, and the reaction is kept for 3 hours;
[0035] (2) After the reaction is completed, distill and concentrate, add the concentrated solution to 700ml of experimental water, stir and cool down to room temperature, let stand, precipitate and then suction filter to obtain the crude product;
[0036] (3) Put the crude product into 220ml of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After concentrated distillation, suction filtration and drying, 54.1 g of pure product was obtained, the yield was about 84.7%, the content was 99.63% by HPLC, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com