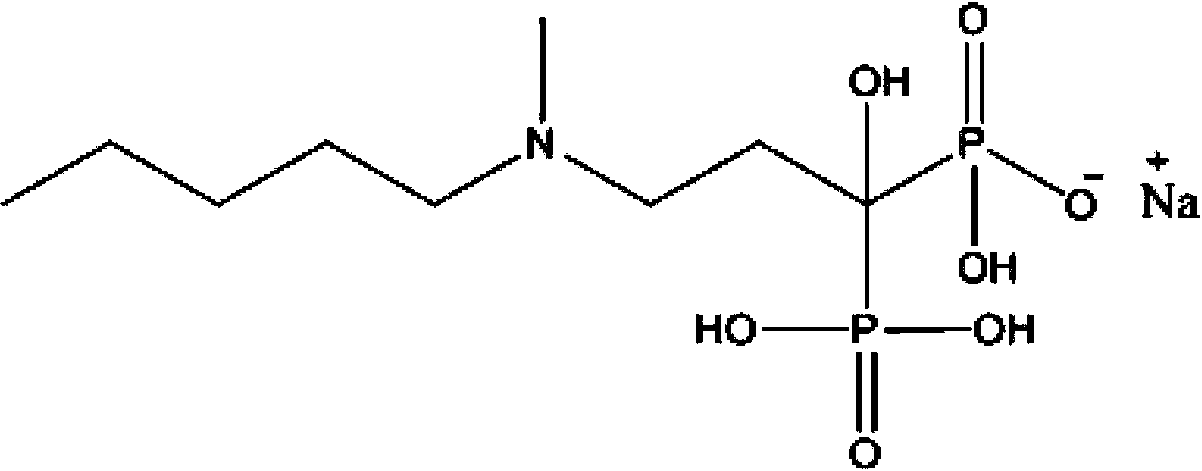
Ibandronate sodium containing injection
A technology of sodium ibandronate and injection, applied in the field of medicine, can solve the problems of irritation, respiratory system irritation, increase the potential cost of products, etc., and achieve the effect of eliminating irritation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
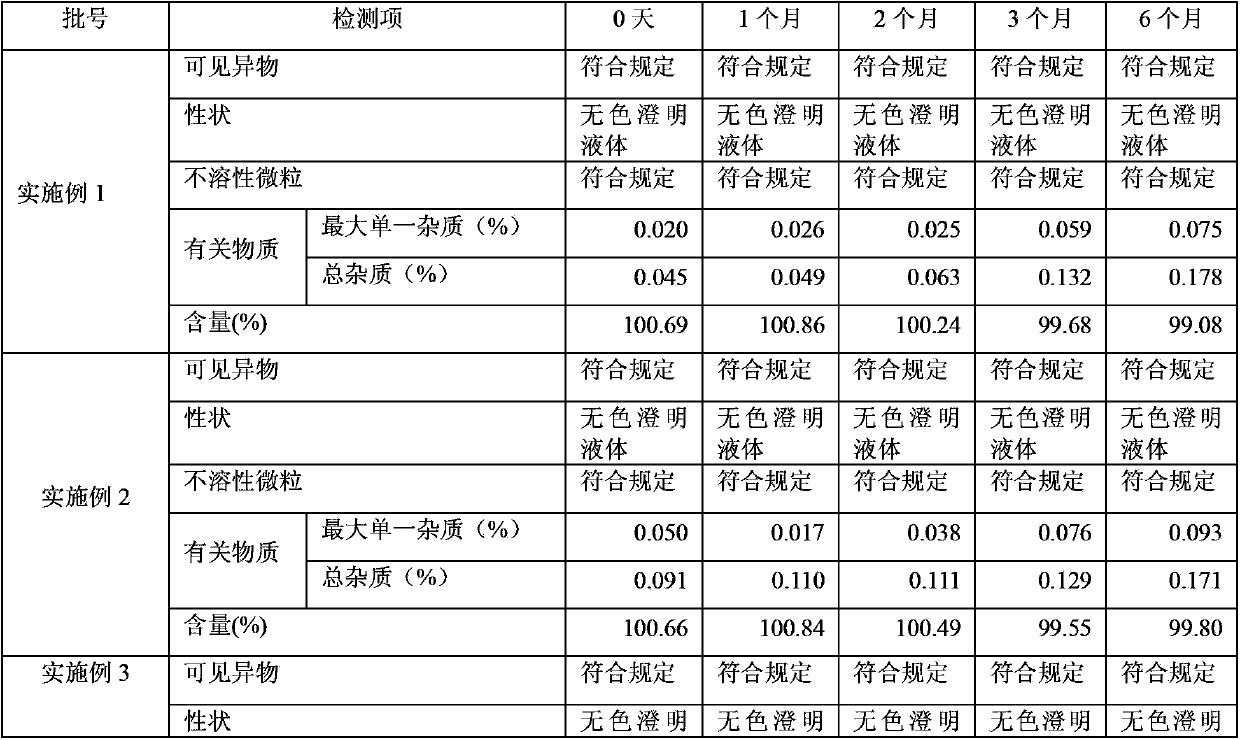
Examples
Embodiment 1
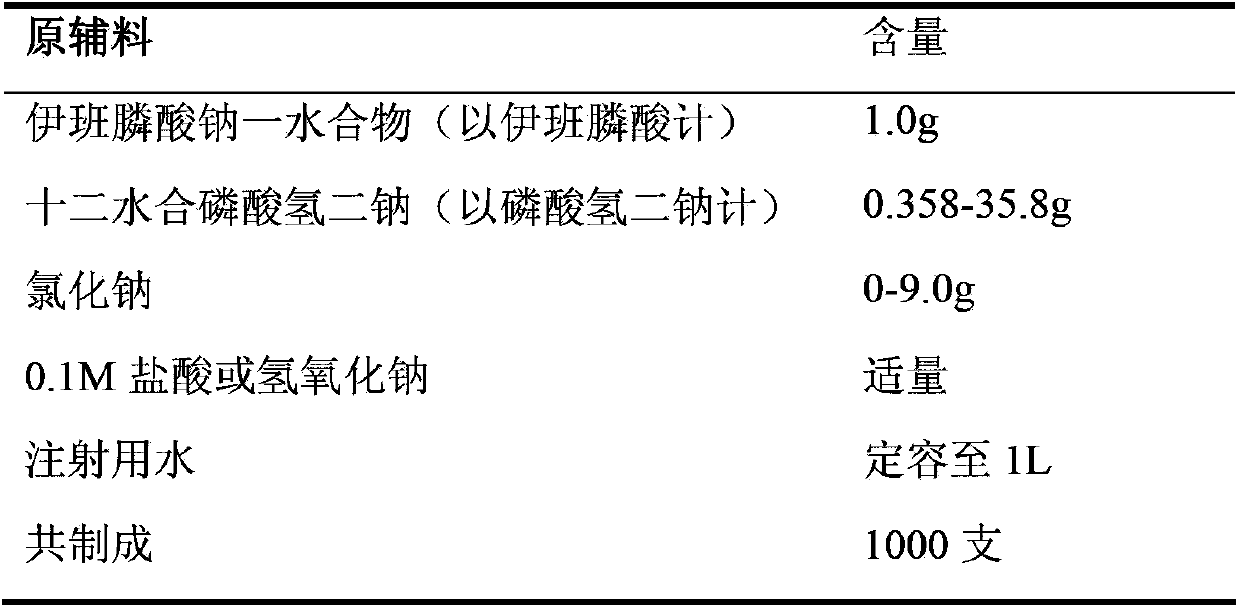
[0041] Embodiment 1: Injection of the present invention
[0042]
[0043] Preparation Process:
[0044] 1) Take 90% of the prescribed amount of water for injection, add the prescribed amount of sodium chloride and disodium hydrogen phosphate dodecahydrate in sequence, and stir to dissolve without heating.
[0045] 2) Add the prescription amount of sodium ibandronate monohydrate to the solution obtained above, stir and dissolve without heating.
[0046] 3) Measure the pH value of the solution obtained above, and adjust the pH value of the solution to 3.5-4.5 with 0.1M hydrochloric acid or sodium hydroxide. Dilute to the total volume with water for injection and stir.
[0047] 4) Add 0.1% activated carbon for needles to the solution obtained above, and stir for 30 minutes.
[0048] 5) Filter the solution obtained above with a 5um titanium filter to remove activated carbon.
[0049] 6) Filter the above solution with a 0.2um plate filter to remove other insoluble particles ...
Embodiment 2
[0054] Embodiment 2: Injection of the present invention
[0055]
[0056] Preparation Process:
[0057] 1) Take 90% of the prescribed amount of water for injection, add the prescribed amount of sodium chloride and disodium hydrogen phosphate dodecahydrate in sequence, and stir to dissolve without heating.
[0058] 2) Add the prescription amount of sodium ibandronate monohydrate to the solution obtained above, stir and dissolve without heating.
[0059]3) Measure the pH value of the solution obtained above, and adjust the pH value of the solution to 3.54.5 with 0.1M hydrochloric acid or sodium hydroxide. Dilute to the total volume with water for injection and stir.
[0060] 4) Add 0.1% activated carbon for needles to the solution obtained above, and stir for 30 minutes.
[0061] 5) Filter the solution obtained above with a 5um titanium filter to remove activated carbon.
[0062] 6) Filter the above solution with a 0.2um plate filter to remove other insoluble particles sm...
Embodiment 3
[0067] Embodiment 3: Injection of the present invention
[0068]
[0069] Preparation Process:
[0070] 1) Take 90% of the prescribed amount of water for injection, add the prescribed amount of disodium hydrogen phosphate dodecahydrate in turn, stir to dissolve, without heating. 2) Add the prescription amount of sodium ibandronate monohydrate to the solution obtained above, stir and dissolve without heating.
[0071] 3) Measure the pH value of the solution obtained above, and adjust the pH value of the solution to 3.54.5 with 0.1M hydrochloric acid or sodium hydroxide. Dilute to the total volume with water for injection and stir.
[0072] 4) Add 0.1% activated carbon for needles to the solution obtained above, and stir for 30 minutes.
[0073] 5) Filter the solution obtained above with a 5um titanium filter to remove activated carbon.
[0074] 6) Filter the above solution with a 0.2um plate filter to remove other insoluble particles smaller than 5um and further increase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com