Method for preparing sodium ibandronate crystal and hydrated crystal thereof
A technology of sodium ibandronate and crystals, applied in the field of crystal preparation of compounds, can solve the problems of unstable composition, unsuitable pharmaceutical composition, unfavorable large-scale industrial manufacturing, etc., and achieve simple and easy manufacturing method and convenient production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
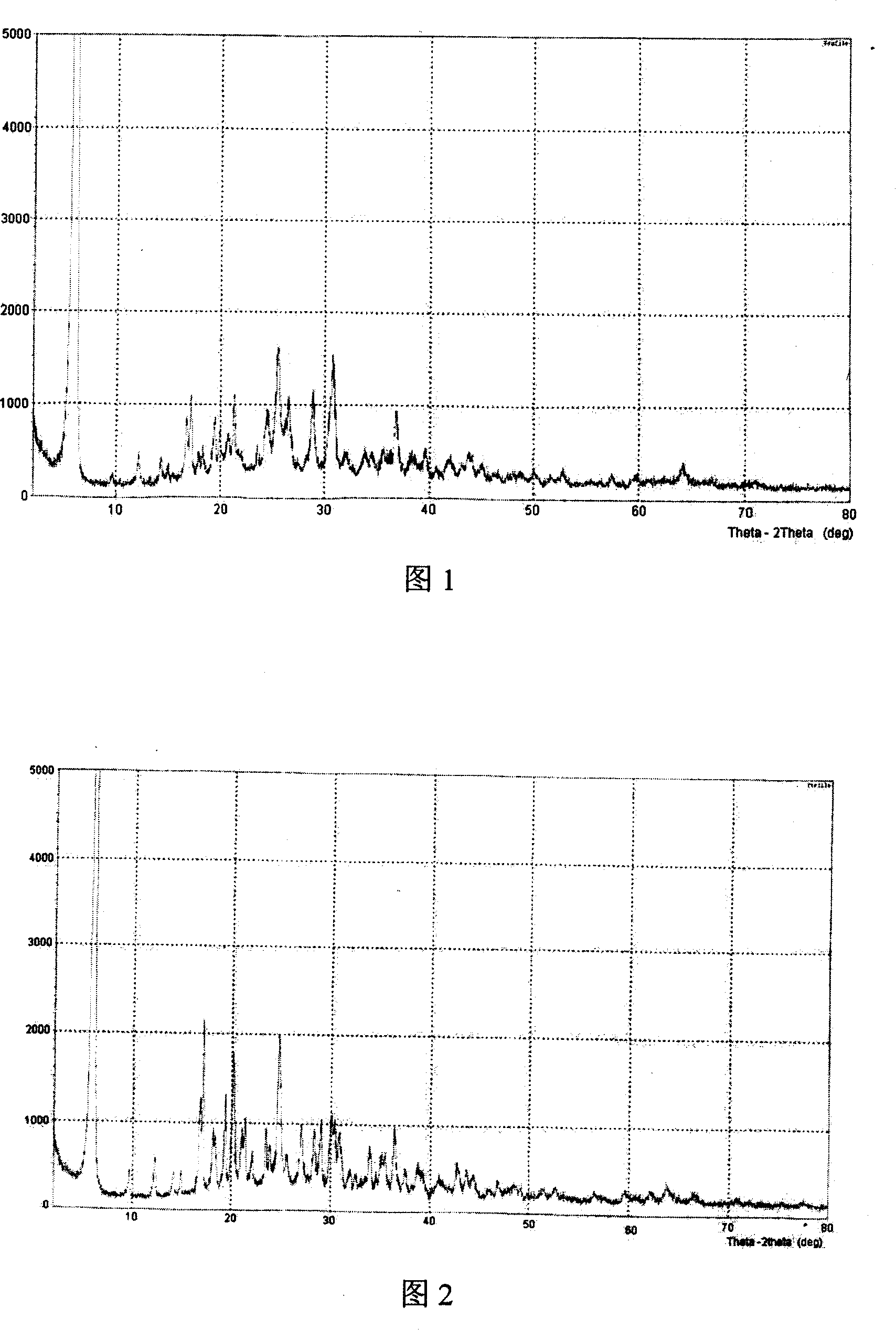
[0024] Add 6g of sodium ibandronate to 40ml of water, stir and dissolve at room temperature, add 80ml of DMF dropwise, a white solid precipitates, continue to stir for 2 hours, cool to 0°C, filter with suction, and vacuum dry at 60-70°C for 3 hours to obtain Ibandronate Sodium phosphonate monohydrate crystalline state (I) 5g, X-ray powder diffraction pattern is shown in Figure 1. The dehydration peak shown by its TGA thermal analysis is 4.52%, and the bulk density is 0.75g / cm3.
Embodiment 2
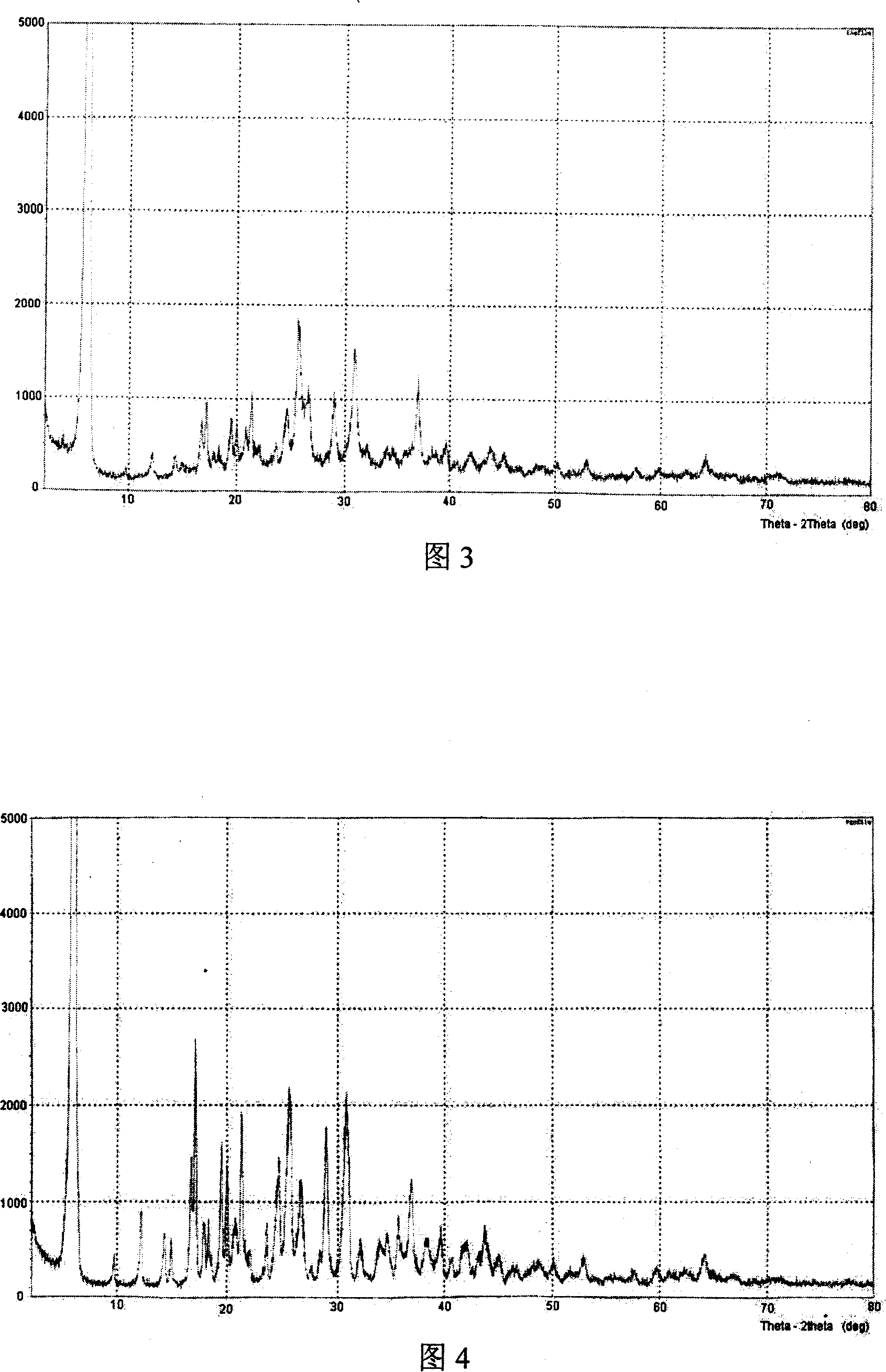
[0026] Add 5g of ibandronic acid into 50ml of water, add 0.6g of solid sodium hydroxide under normal temperature stirring, stir to form a homogeneous solution, add 80ml of DMF at one time, a white solid precipitates out, continue to stir for 2 hours, cool to 0°C, filter with suction, and Vacuum-dried at 60-70°C for 3 hours to obtain 4.8 g of ibandronate sodium monohydrate crystalline state (I).
Embodiment 3
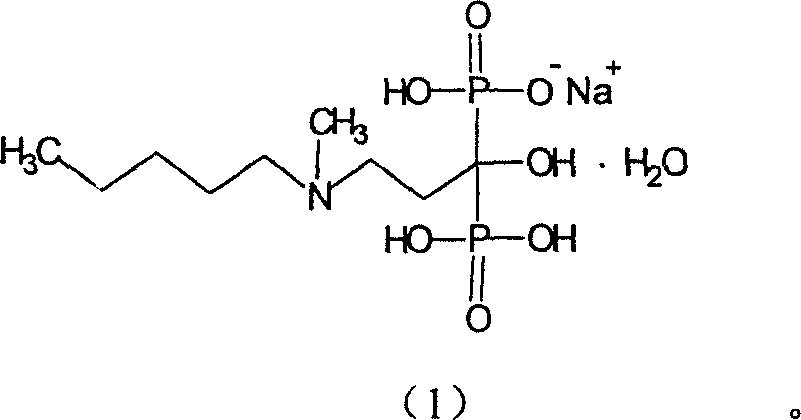
[0028] Sodium ibandronate 6g was added to 40ml of water, stirred and dissolved at room temperature, 80ml of dimethylacetamide was added dropwise, and a white solid was precipitated, continued to stir for 2 hours, cooled to 0°C, filtered with suction, and vacuum-dried at 60-70°C for 3 hours to obtain Sodium ibandronate monohydrate crystalline state (I) 5.2g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com