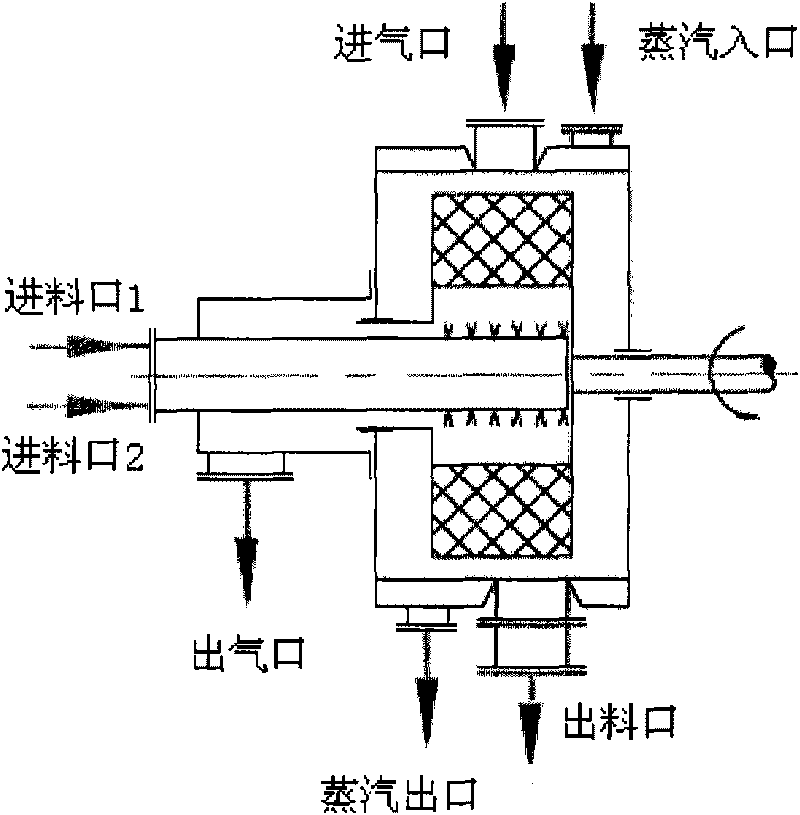
Method for preparing nano iron phosphate
A nano-ferric phosphate and phosphoric acid technology, which is applied in the fields of nanostructure manufacturing, nanotechnology, nanotechnology, etc., achieves the effects of simple equipment, low preparation cost and small size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Adjust the rotational speed of the rotating packed bed to 500r·min -1 , the 0.1mol·L -1 Mixed solution of ferric nitrate and phosphoric acid in 0.1L·min -1 The speed is input into the rotating packed bed through the metering pump, and at the same time, 0.22mol L is input with the metering pump -1 The ammonia solution is used to control the pH value of the reaction system to 1.70, and the reaction temperature is 60°C. The raw material solution is quickly and fully mixed under the action of large centrifugal force, and the reaction crystallizes to form nano-iron phosphate particles. After the reaction, the mixed solution is discharged from the rotating packed bed. The outlet is discharged, and the nano-scale ferric phosphate is obtained through post-treatment processes such as filtration, washing, and drying.
Embodiment 2
[0033] Adjust the rotational speed of the rotating packed bed to 2000r·min -1 , the 0.5mol·L -1 Ferric sulfate and sodium dihydrogen phosphate and 0.05g·L -1 The mixed solution of sodium dodecylsulfonate at 0.2L·min -1 The speed is input into the rotating packed bed through the metering pump, and at the same time, 2.0mol L is input with the metering pump -1 The sodium hydroxide solution is used to control the pH value of the reaction system to 3.00, and the reaction temperature is 15°C. The raw material solution is quickly and fully mixed under the action of a large centrifugal force, and the reaction crystallizes to form nano-iron phosphate particles. It is discharged from the discharge port, and the nano-scale ferric phosphate is obtained through post-treatment processes such as filtration, washing, and drying.
Embodiment 3
[0035] Adjust the rotational speed of the rotating packed bed to 1500r·min -1 , the 1.0mol·L -1 Iron acetate and phosphoric acid 0.1g·L -1 The mixed solution of polyethylene glycol and 1.0L·min -1 The speed is input into the rotating packed bed through the metering pump, and at the same time, 2.0mol L is input with the metering pump -1Potassium hydroxide solution is used to control the pH value of the reaction system to 5.80, and the reaction temperature is 45°C. The raw material solution is quickly and fully mixed under the action of large centrifugal force, and the reaction crystallizes to form nano-iron phosphate particles. It is discharged from the discharge port, and the nano-scale ferric phosphate is obtained through post-treatment processes such as filtration, washing, and drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| rotating speed | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com