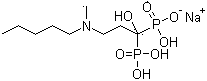
Sodium ibandronate injection composition
A kind of ibandronate sodium, injection technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
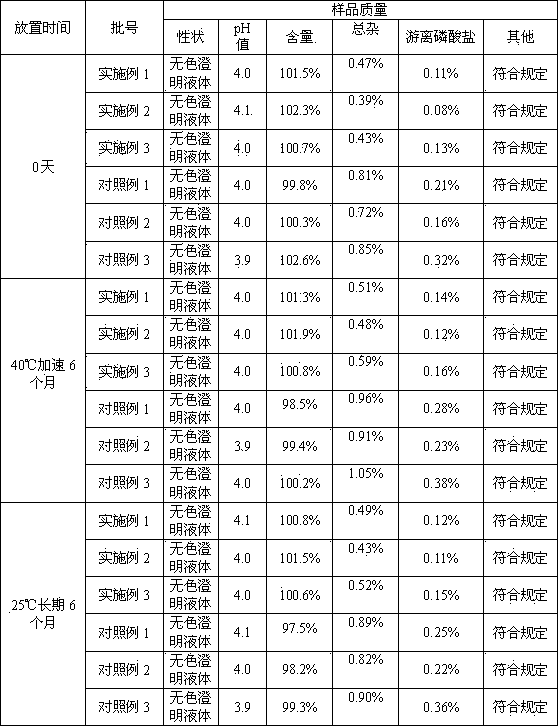
Embodiment 1
[0042] Prescription: ibandronate sodium 6.75g
[0044]Sodium metabisulfite 9.00g
[0045] Sodium acetate 4.8g
[0046] Acetic acid appropriate to pH 4
[0047] Add water for injection to 6000ml
[0048] Made 1000 pieces
[0049] Take the needle and add activated carbon to sodium hydroxide solution, raise the temperature to 50°C, stir for 1 hour, filter, wash the residue with dilute hydrochloric acid solution, and then rinse with water for injection 2-5 times until it becomes neutral, and dry it for later use.
[0050] Dissolve sodium chloride and sodium acetate in 95% of the prescribed amount of water for injection, stir to dissolve and adjust the pH to 4.0 with acetic acid; add the prescribed amount of sodium ibandronate, stir to dissolve, make up the water for injection to the full amount; 0.03% spare activated carbon, heat preservation and stirring for 15 minutes, primary filtration, fine filtration, cooling to room temperature, determinati...
Embodiment 2
[0057] Prescription: ibandronate sodium 6.75g
[0058] Sodium chloride 42g
[0059] Sodium metabisulfite 6.75g
[0060] Sodium acetate 4.8g
[0061] Acetic acid appropriate to pH 5
[0062] Add water for injection to 6000ml
[0063] Made 3000 pieces
[0064] Take the needle and add activated carbon to sodium hydroxide solution, raise the temperature to 50°C, stir for 1 hour, filter, wash the residue with dilute hydrochloric acid solution, and then rinse with water for injection 2-5 times until it becomes neutral, and dry it for later use.
[0065] Dissolve sodium chloride and sodium acetate in 90% of the prescribed amount of water for injection, stir to dissolve and adjust the pH to 5.0 with acetic acid; add the prescribed amount of sodium ibandronate, stir to dissolve, make up the water for injection to the full amount; 0.03% spare activated carbon, heat preservation and stirring for 15 minutes, primary filtration, fine filtration, cooling to room temperature, determinat...
Embodiment 3
[0072] Prescription: ibandronate sodium 6.75g
[0073] Sodium chloride 67.5g
[0074] Sodium metabisulfite 13.50g
[0075] Sodium acetate 4.8g
[0076] Acetic acid Appropriate to pH 6
[0077] Add water for injection to 6000ml
[0078] Made 6000 pieces
[0079] Take the needle and add activated carbon to sodium hydroxide solution, raise the temperature to 50°C, stir for 1 hour, filter, wash the residue with dilute hydrochloric acid solution, and then rinse with water for injection 2-5 times until it becomes neutral, and dry it for later use.
[0080] Dissolve sodium chloride and sodium acetate in 80% of the prescribed amount of water for injection, stir to dissolve and adjust the pH to 6.0 with acetic acid; add the prescribed amount of sodium ibandronate, stir to dissolve, make up the full amount of water for injection; 0.03% spare activated carbon, heat preservation and stirring for 15 minutes, primary filtration, fine filtration, cooling to room temperature, determinat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com