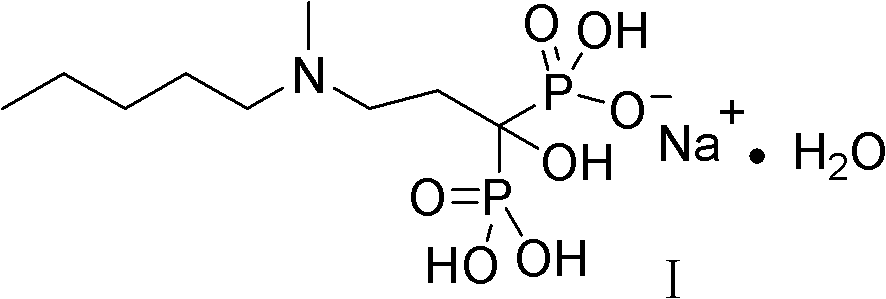
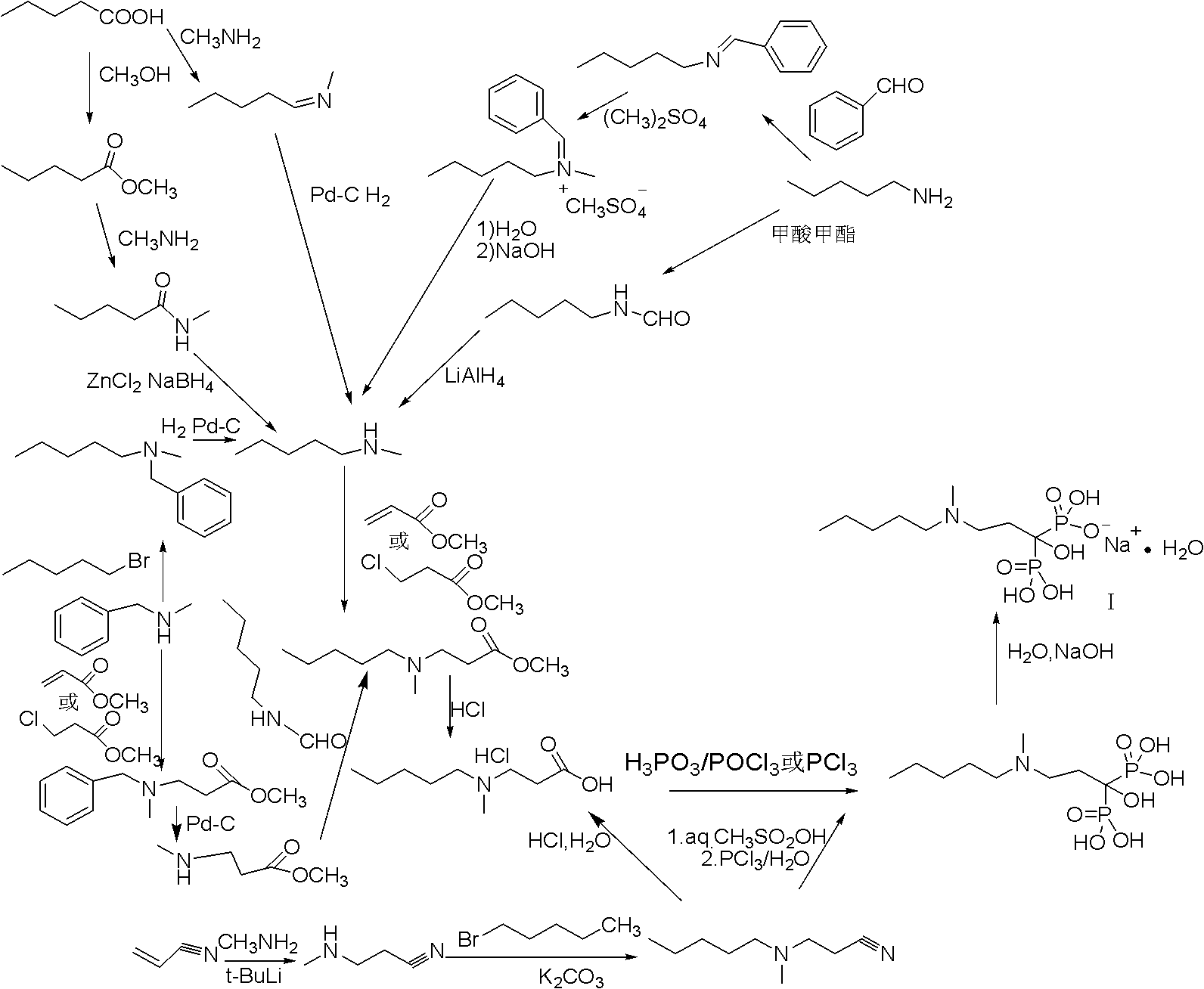
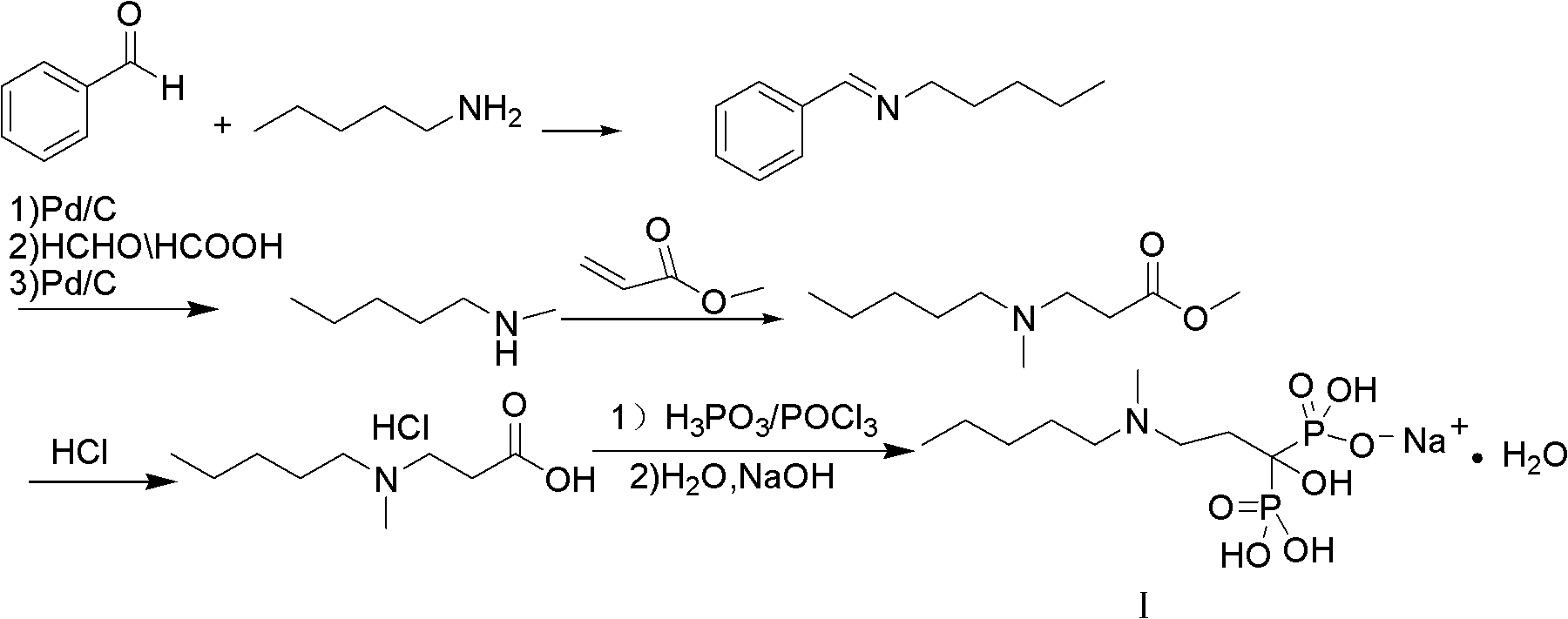
Method for synthesizing sodium ibandronate
A technology of sodium ibandronate and alkyl ibandronate, which is applied in a new synthesis field, can solve the problems of cumbersome operation and long preparation route, and achieve the effect of improving safety, less steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The following generally describes the preparation of ibandronate sodium
[0037] 1. Synthesis of N-methyl-β-alanine methyl ester
[0038] Add methylamino alcohol solution to the reaction device and slowly add methyl acrylate dropwise at -10--5°C. After the dropwise addition, continue to react at -10--5°C for 24 hours. After the reaction is completed, remove it under reduced pressure at room temperature Solvent and unreacted methylamine, the residue was distilled under reduced pressure, and the distillation conditions were 40-50°C, 4-5 torr fractions were collected.
[0039] 2. Synthesis of [1-hydroxy-3-methylaminopropylene] bisphosphate tetraalkyl ester
[0040]
[0041] Add N-methyl-β-alanine methyl ester, formula (V) compound, dialkyl phosphinate, reaction solvent in the reaction device, heat and reflux for reaction, after the reaction is completed, add water to stir, and pour the liquid into the liquid funnel, extracted with ethyl acetate, collected the organic ...
Embodiment 1
[0045] Embodiment 1: bisphosphonic acid monosodium salt
[0046]1. Synthesis of N-methyl-β-alanine methyl ester
[0047] Add methylamino alcohol solution (150ML, 1.5mol) into a 500ml reaction flask and slowly add methyl acrylate (75g, 0.87mol) dropwise at -10--5°C. The reaction was continued for 24 hours at low temperature. After the reaction was completed, the solvent and unreacted methylamine were removed under reduced pressure at room temperature, and the residue was distilled under reduced pressure. The distillation conditions were 40-45 ° C, 86 g of fractions at 4-5 torr, and the yield was 84.4%. 98.2% purity.
[0048] 2. Synthesis of tetramethyl [1-hydroxy-3-methylaminopropylene] diphosphate
[0049] In a 1000ml reaction flask, add 86g (0.734mol) of N-methyl-β-alanine methyl ester, 5g of sodium methoxide, dimethyl phosphinate (177.7g, 1.615mol), 250ml of anhydrous methanol, and heat to reflux React for 3 hours, the reaction is over, add 300ml of water, stir for 0.5 ho...
Embodiment 2
[0055] 1. Synthesis of [1-hydroxy-3-methylaminopropylene] tetraethyl diphosphate
[0056] Add 86 g (0.734 mol) of N-methyl-β-alanine methyl ester, 7 g sodium ethoxide, diethyl phosphinate (262.2 g, 1.9 mol), and 200 ml absolute ethanol into a 1000 ml reaction flask, and heat to reflux React for 3 hours, the reaction is over, add 280ml of water, stir for 0.5 hours, pour the liquid into a separatory funnel, extract with 300ml of ethyl acetate, collect the organic layer, wash with 200ml of saturated brine, and concentrate the organic phase under reduced pressure to obtain [1- 198 g of tetraethyl hydroxy-3-methylaminopropylene] diphosphate, the yield is 74.7%.
[0057] 2. Synthesis of [1-hydroxyl-3-(N-methyl-N-n-pentylamino)propylene]bisphosphonic acid monosodium salt monohydrate (sodium ibandronate)
[0058] Pour 198 g (0.55 mol) of [1-hydroxy-3-methylaminopropylene] tetraethyl diphosphate into the reaction flask, add 200 ml of ethanol, heat to reflux, and slowly drop in 63.95 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com