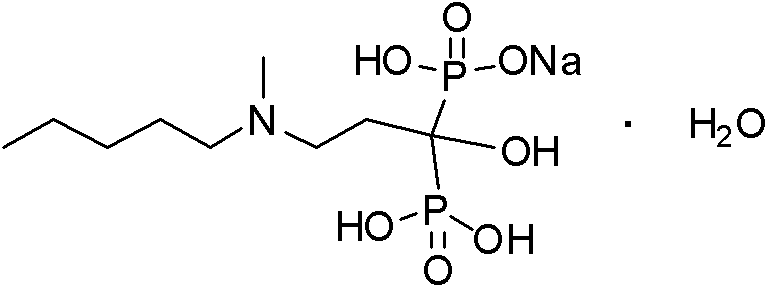
Method for preparing sodium Ibandronate monohydrate
A technology of sodium ibandronate and monohydrate, which is applied in the field of preparation of sodium ibandronate monohydrate, can solve the problems of extremely high equipment and environmental requirements, unsuitability for industrial production, difficult operation, etc., and achieve the goal of reaction process Safe and reliable, easy to industrialized production, good reaction selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
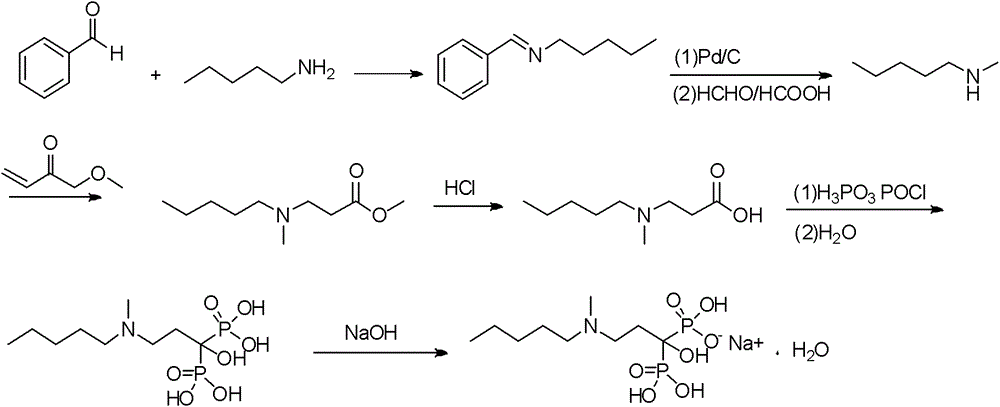
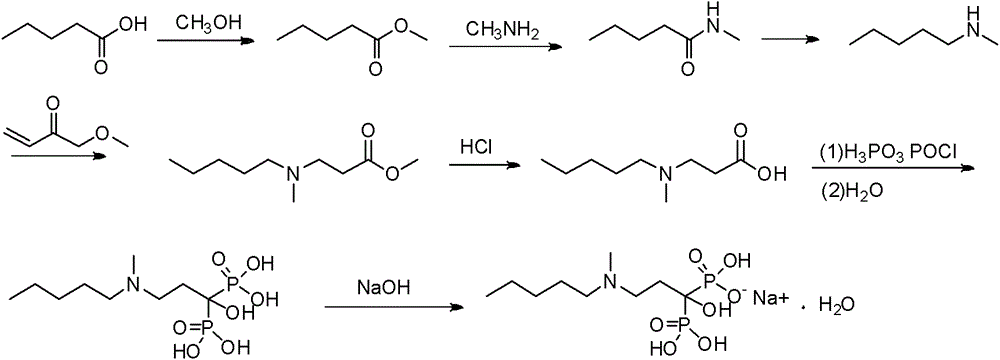
[0054] 1) A 50L dry and clean double-layer glass reaction kettle is connected to a hydrochloric acid gas absorption device, and at 25-30°C, add 3kg (14.3mol) of 3-[methyl(pentyl)amino]propionate and 4.4kg of phosphorous acid (53.57mol) and DMI9L, stir for 10min, drop in phosphorus trichloride, control the system temperature at 60-70°C, after the drop-in is complete, raise the temperature to 80°C for 6h, cool to 30°C, drop in 20L of deionized water, control The temperature of the system is < 45°C, after the drop is completed, heat and stir until reflux, the temperature is 110°C, react for 7 hours, TLC controls the end point (developing agent: water-glacial acetic acid-n-butanol=1:1:3), the reaction is complete, Cool to 40°C, add 30% sodium hydroxide solution dropwise to adjust the pH to 4.1-4.3, add 20L of methanol, stir for 2 hours, centrifuge and filter, wash the solid with 2L of methanol, and dry under reduced pressure at 55-60°C to obtain ibandronic acid as a white solid So...
Embodiment 2
[0069] 1) A 50L dry and clean double-layer glass reaction kettle is connected to a hydrochloric acid gas absorption device, and at 25-30°C, add 3kg (14.3mol) of 3-[methyl(pentyl)amino]propionate and 4.4kg of phosphorous acid (53.57mol) and DMI12L, stir for 10min, drop in phosphorus trichloride, control the system temperature at 60-70°C, after the drop-in is completed, raise the temperature to 80°C for 6h, cool to 30°C, drop in 20L of deionized water, control The temperature of the system is < 45°C, after the drop is completed, heat and stir until reflux, the temperature is 110°C, react for 7 hours, TLC controls the end point (developing agent: water-glacial acetic acid-n-butanol=1:1:3), the reaction is complete, Cool to 40°C, add 30% sodium hydroxide solution dropwise to adjust the pH to 4.1-4.3, add 20L of methanol, stir for 2 hours, centrifuge and filter, wash the solid with 2L of methanol, and dry under reduced pressure at 55-60°C to obtain ibandronic acid as a white solid ...
Embodiment 3
[0073] 1) A 50L dry and clean double-layer glass reaction kettle is connected to a hydrochloric acid gas absorption device, and at 25-30°C, add 3kg (14.3mol) of 3-[methyl(pentyl)amino]propionate and 4.4kg of phosphorous acid (53.57mol) and DMI3L, stir for 10min, add 12L of toluene, drop in phosphorus trichloride, control the temperature of the system at 60-70°C, after the addition is complete, raise the temperature to 80°C for 7h, cool to 30°C, and discard the organic layer , the residue was dropped into 20L of deionized water, and the temperature of the system was controlled at <45°C. After the drop was completed, heated and stirred to reflux at a temperature of 105°C, reacted for 5h, and controlled the end point by TLC (developing agent: water-glacial acetic acid-n-butanol =1:1:3), after the reaction is completed, cool to 40°C, add dropwise 30% sodium hydroxide solution to adjust the pH to 4.1~4.3, add 20L methanol, stir for 2h, centrifuge, wash the solid with 2L methanol, 55...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com