Preparation method of sodium ibandronate monohydrate
A technology of sodium ibandronate and monohydrate, applied in the field of preparation of sodium ibandronate monohydrate, to achieve the effects of avoiding genotoxic substances, high yield, avoiding patent protection and solvation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
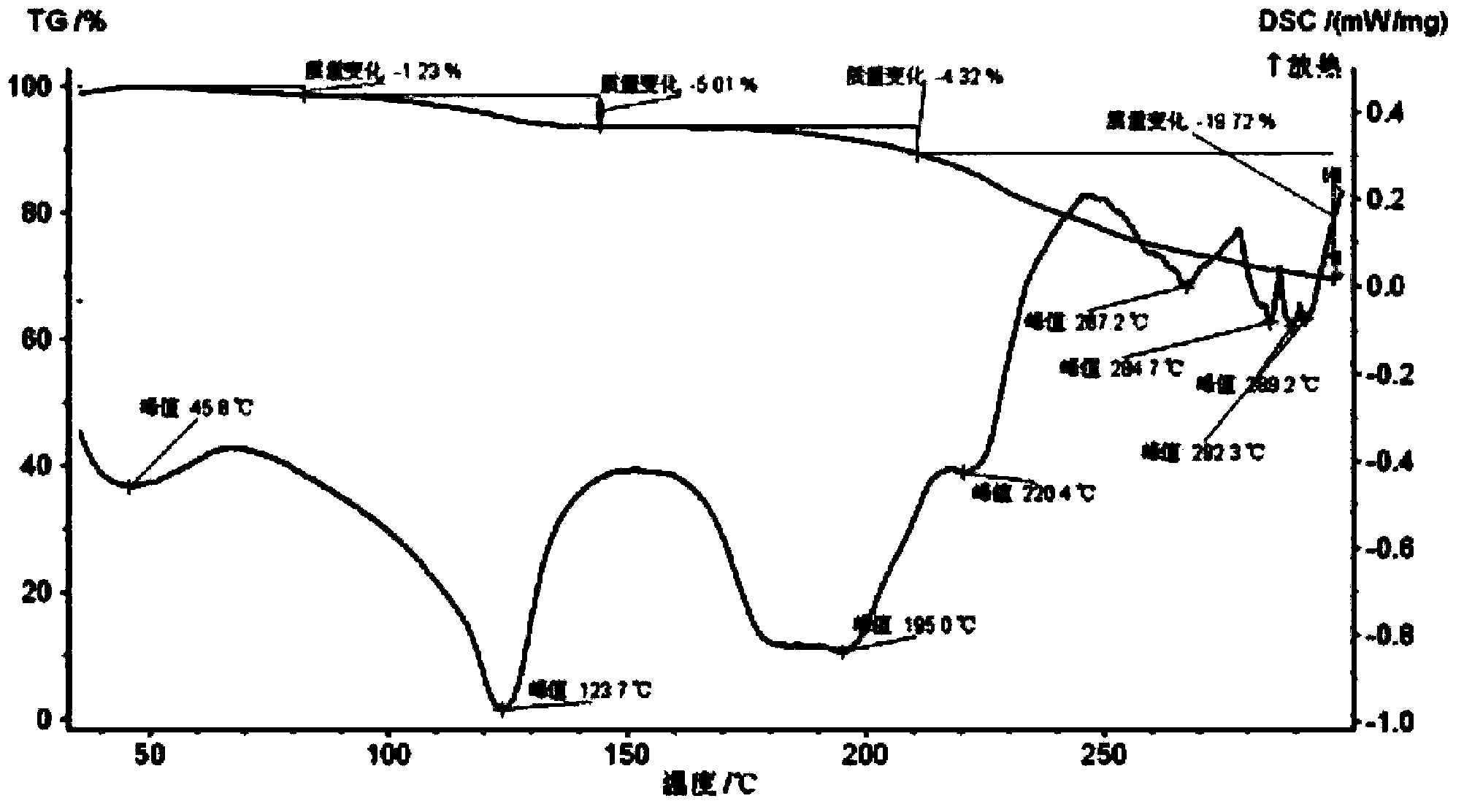
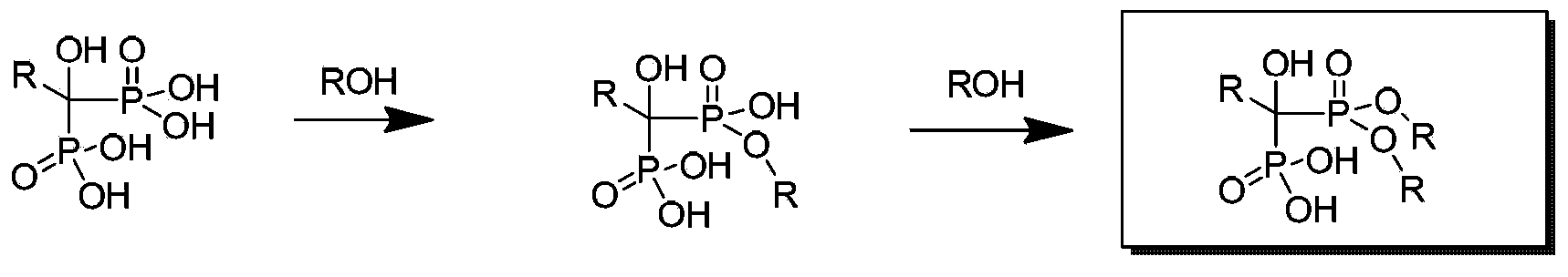
Image
Examples
Embodiment 1
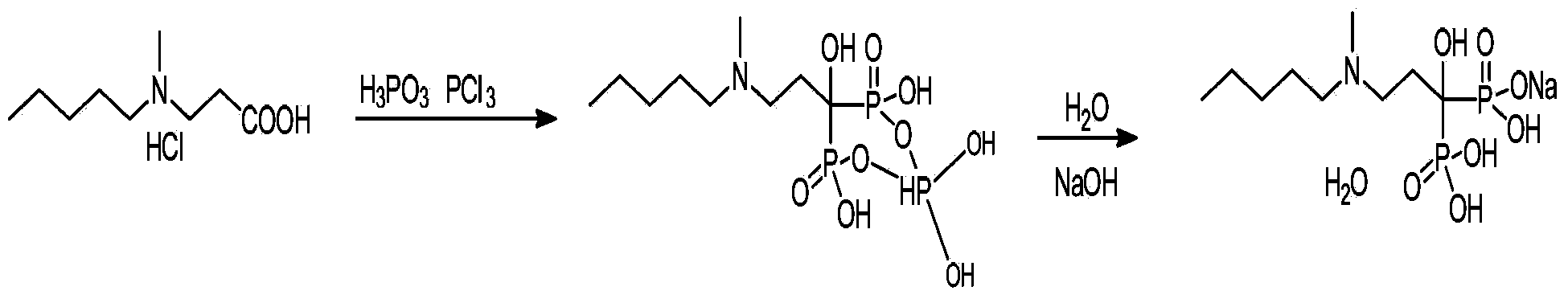
[0019] Add 40.0g of aminopropionate hydrochloride, 31.0±1g of phosphite, and 153.0g of toluene into the reactor, heat to T=70-75°C, keep stirring at this temperature and slowly add 26.0g of phosphorus trichloride dropwise. T=85-95°C Stir the reaction to complete, slowly lower the temperature of the reactor to T=20-40°C, separate and discard the toluene layer. Then add toluene into the reactor, stir, and discard the toluene layer. Drinking water was added to the reactor, and the reaction was stirred for an hour. The filtrate was concentrated to give an oil. Add purified water to the oil, and slowly add saturated sodium hydroxide solution to adjust the pH value of the solution to 4.0-4.6. Acetone was slowly added dropwise, a large amount of solids precipitated, the material was put into a suction filtration funnel, and suction filtered to obtain crude ibandronate sodium.
[0020] Add the crude sodium ibandronate and purified water (crude sodium ibandronate: purified water = 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com