Solid and crystalline ibandronate sodium and processes for preparation thereof
A technology of sodium ibandronate and crystal form, applied in the field of solid state chemistry of sodium ibandronate, can solve the problems of restricting oral active ingredients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
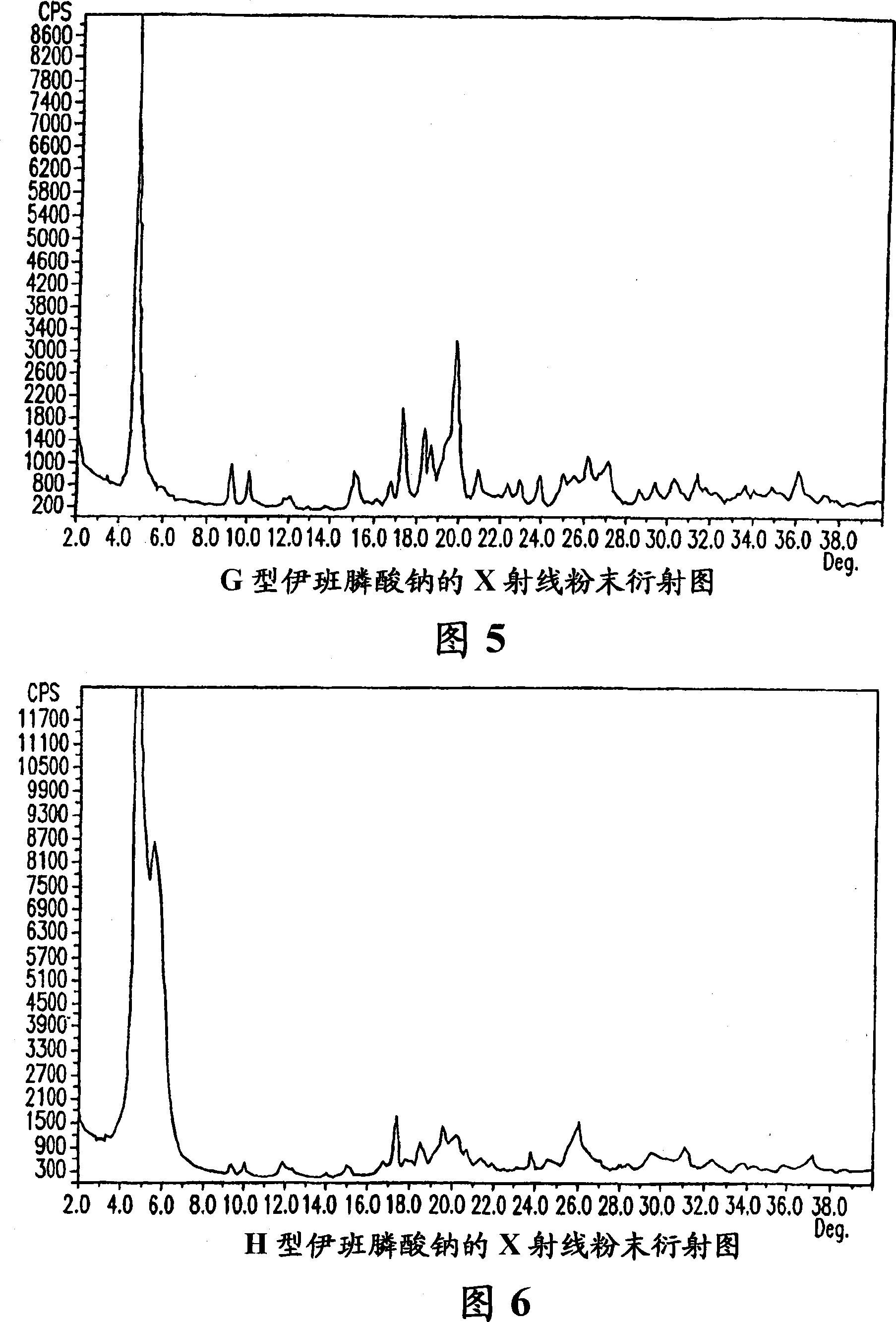
Image
Examples
Embodiment 1
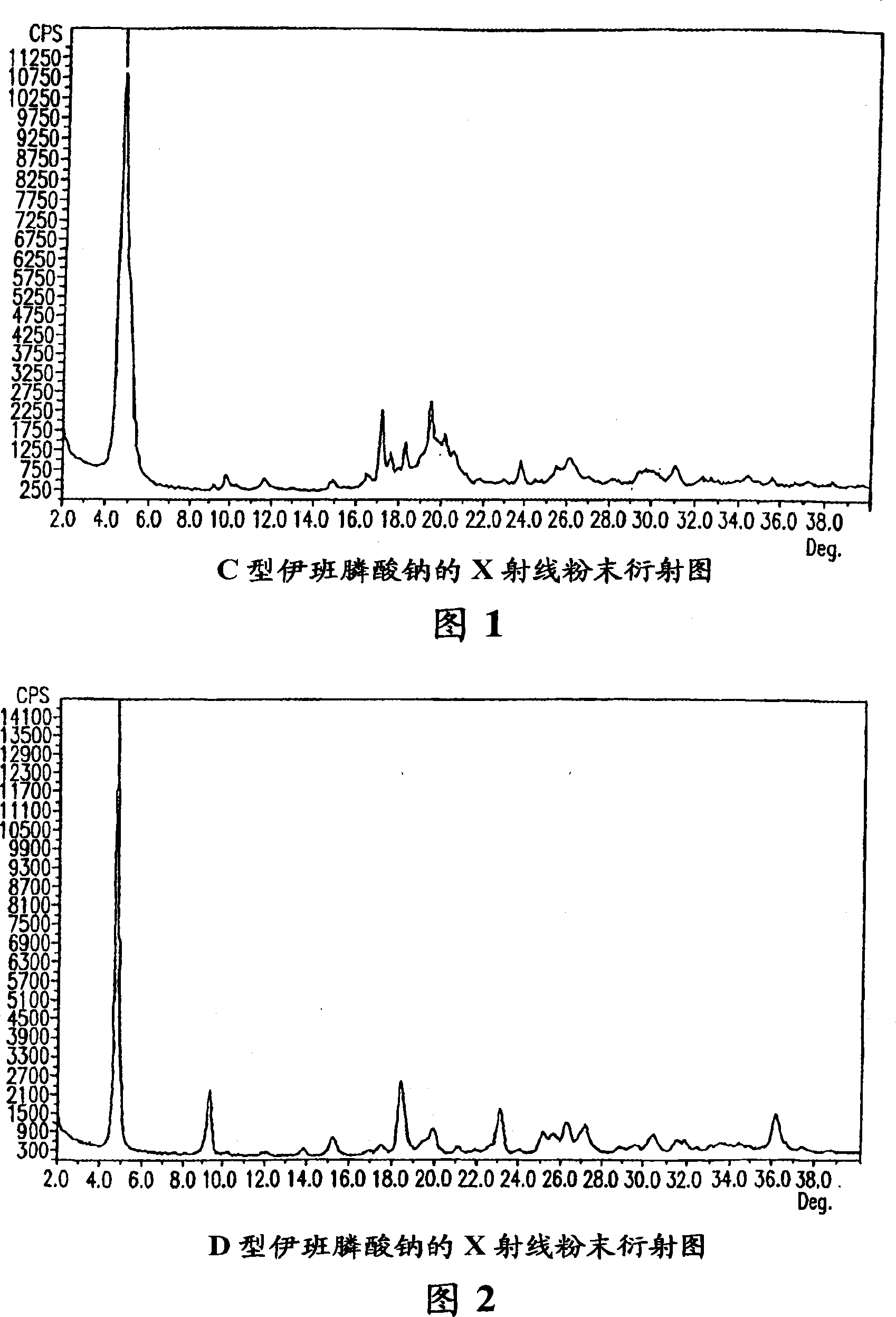
[0187] Sodium ibandronate (3 g) was dissolved in dimethylsulfoxide (DMSO) (20 mL) at 125°C. To the resulting solution was added dropwise 2-butanol (40 mL) to give a white precipitate. The slurry was stirred at 125°C for 3 hours, then cooled to room temperature and stirred for 16 hours. The precipitate was separated by vacuum filtration, washed with 2-butanol (2×5 ml), and dried in a vacuum oven at 50° C. for 24 hours to obtain 3 g of sodium ibandronate in crystal form C. Form C ibandronate sodium was analyzed by TGA as described above, and the weight loss was about 15% to about 16%.
Embodiment 2
[0189] Sodium ibandronate (3 g) was dissolved in DMSO (20 mL) at 120°C. To the resulting solution was added dropwise 1-butanol (40 mL) to give a white precipitate. The slurry was stirred at 120°C for 3 hours, then cooled to room temperature and stirred for 16 hours. The precipitate was isolated by vacuum filtration, washed with 1-butanol (2×5 ml), and dried in a vacuum oven at 50° C. for 24 hours to obtain 3 g of sodium ibandronate in crystal form C.
[0190] Sodium Ibandronate Form D
Embodiment 3
[0192] Sodium ibandronate (3 g) was dissolved in water (6 mL) at reflux temperature. To the resulting solution was added dropwise acetone (50 mL) at reflux temperature to obtain a white precipitate. The slurry was stirred at reflux temperature for 4.5 hours, then cooled to room temperature. The precipitate was separated by vacuum filtration, washed with acetone (3×13 ml), and dried in a vacuum oven at 50° C. for 22 hours to obtain 3.3 g of ibandronate sodium ibandronate in crystal form D. D-type ibandronate sodium was analyzed by TGA, and the weight loss was about 25%.
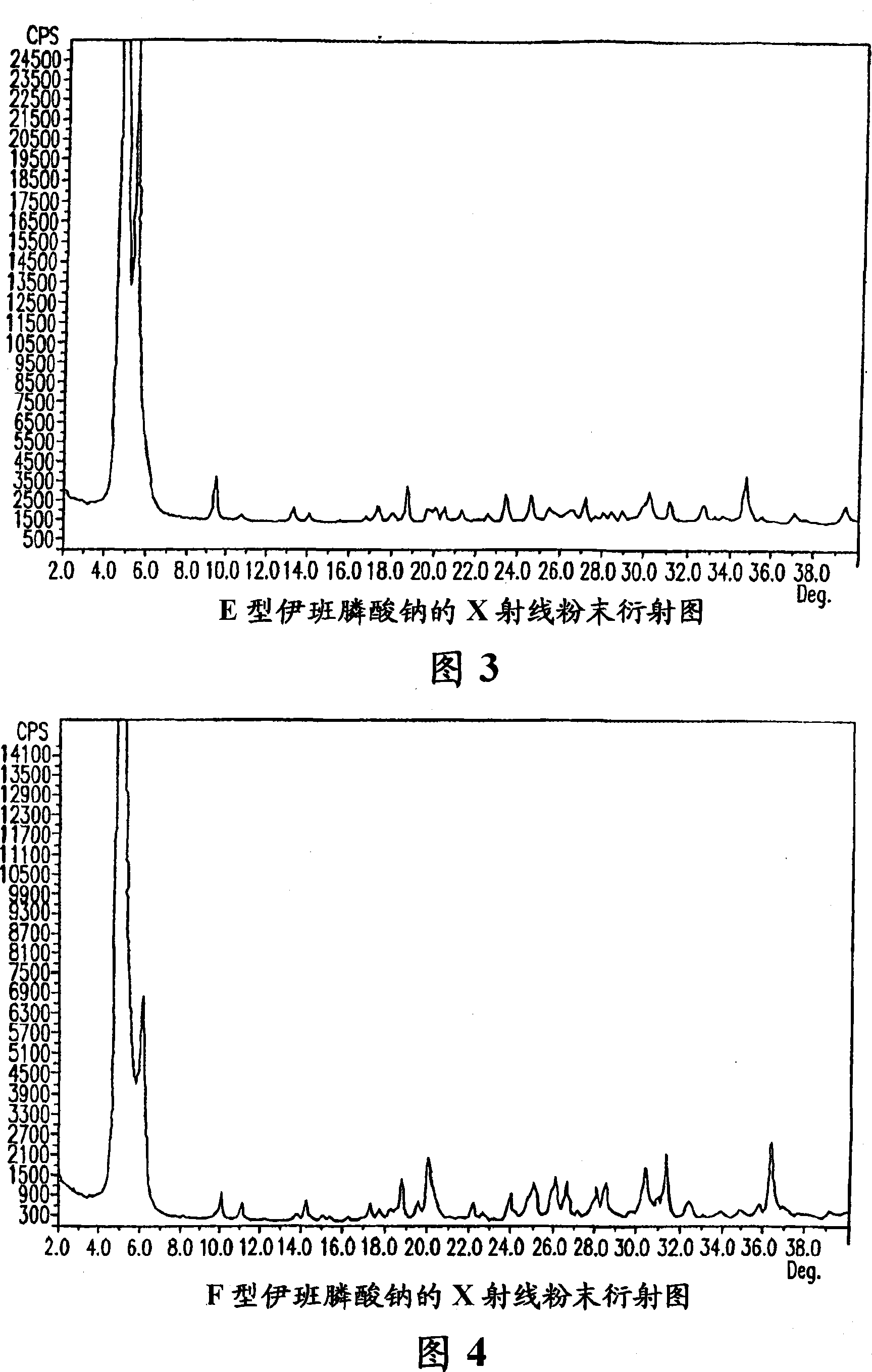
[0193] Sodium Ibandronate Form E
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com