Schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof
A technology of recombinant protein and schistosomiasis, applied in the direction of recombinant DNA technology, application, genetic engineering, etc., to achieve the effect of good antigenicity and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
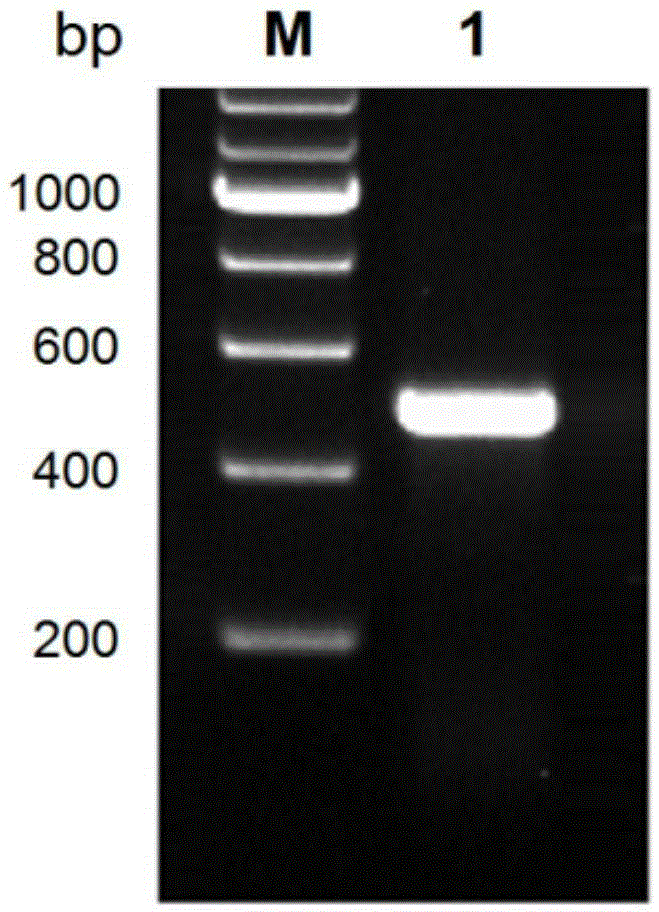
[0045] Cloning of embodiment 1 Schistosoma japonicum SjSAPLP4 gene
[0046] According to the sequence of the SjSAPLP4 gene (GenBankFN315317.1) (sequence shown in SEQ ID NO.1), primers were designed and restriction restriction sites were introduced, as follows:
[0047] PF: 5'- GGATCC AACCACACTGAGTTGGACAT-3' (as shown in SEQIDNO.3, the italic part represents the protective base of the upstream primer, and the underlined part is the BamHI restriction site of the upstream primer);
[0048] PR: 5'- CTCGAG TGGGCATAACTGTATTGTCT-3' (as shown in SEQ ID NO.4, the italic part represents the protective base of the downstream primer, and the underlined part is the XhoI restriction site of the downstream primer);
[0049] Specific primers were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.
[0050] Using the cDNA of 42-day-old male and female adults of Schistosoma japonicum as a template, perform PCR reaction to amplify the ORF fragment of SjSAPLP4 gene. The reaction syste...
Embodiment 2
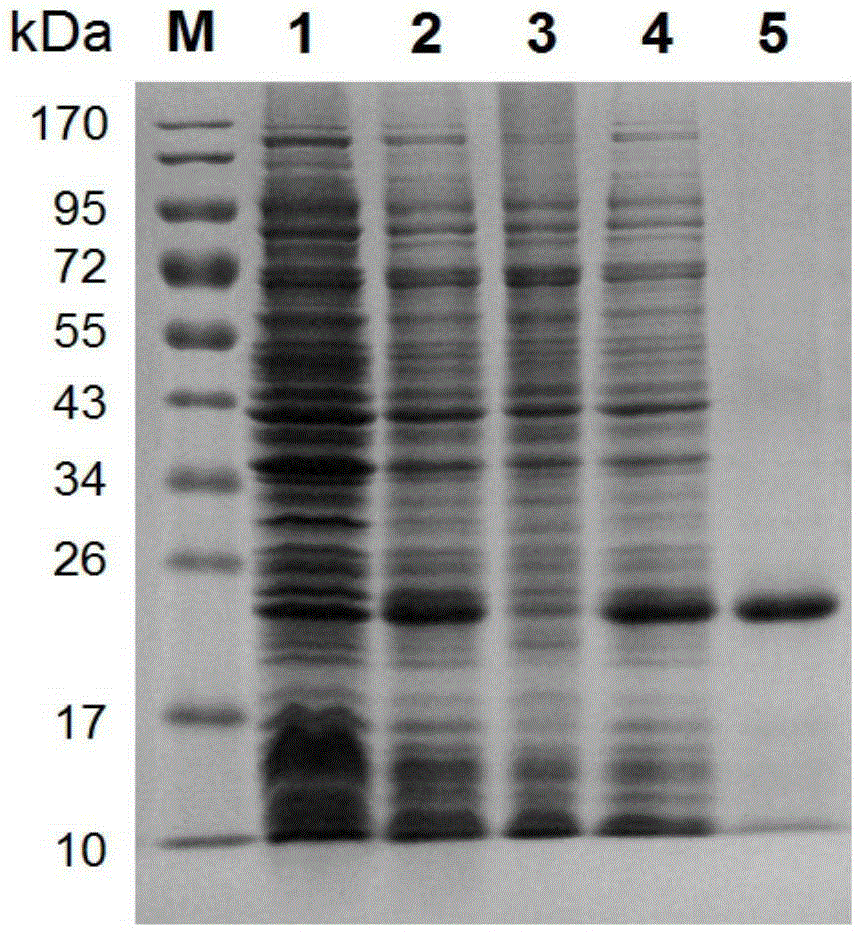
[0054] Example 2 Expression and purification of Schistosoma japonicum SjSAPLP4 recombinant protein
[0055] The pET28a(+)-SjSAPLP4 recombinant plasmid with correct sequencing was transformed into expression competent cells Transetta (DE3) (Beijing Quanshijin Biotechnology Co., Ltd.); the clones identified as positive by PCR were inoculated into LB liquid medium (containing 50 μg / ml kanamycin) 15mL, cultivate overnight at 37°C, transfer 10mL medium into 1L LB medium (containing 50μg / ml kanamycin) the next day, and continue to culture until OD 600nm When the value is 0.8, IPTG with a final concentration of 1 mM was added to induce expression for 4 hours, the cells were collected by centrifugation, and frozen at -80°C for future use.
[0056] Take a small amount of bacteria before induction and after induction and resuspend in PBS buffer, add SDS-PAGE loading buffer, mix well, boil in boiling water bath for 5min to denature the protein. 10 μl of the pre-induction and post-induc...
Embodiment 3
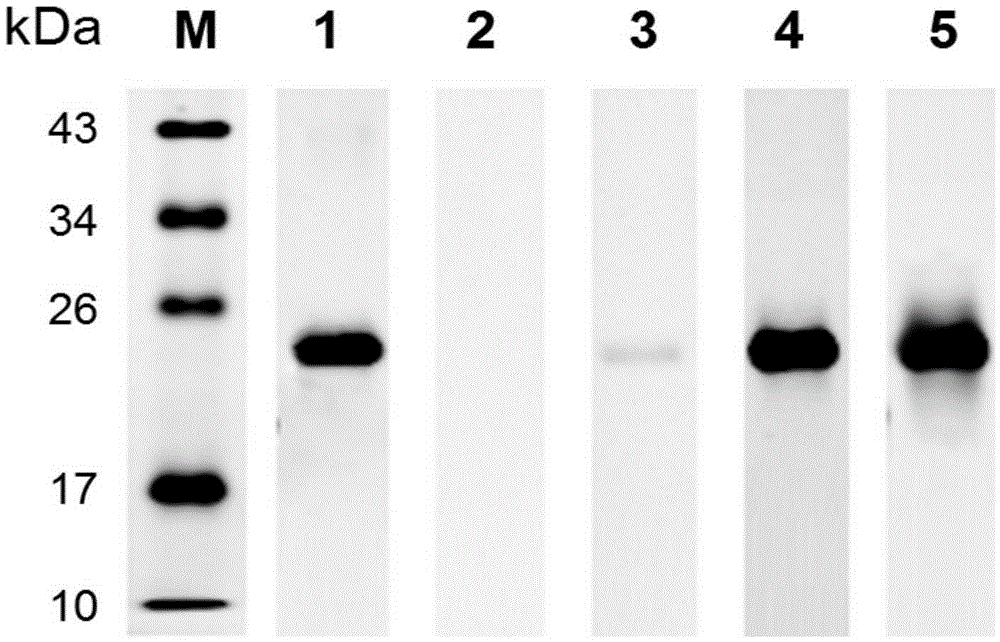
[0058] Example 3 Antigenic Detection of Schistosoma japonicum SjSAPLP4 Recombinant Protein
[0059] SDS-PAGE electrophoresis: 100 ng of the SjSAPLP4 recombinant protein obtained in Example 2 was loaded on the sample, and the electrophoresis conditions were: 100V20min, 120V1h.
[0060] Membrane transfer: The protein in the PAGE gel was transferred to the PVDF membrane by wet transfer method, and the electroporation conditions were: ice bath, 100V1h.
[0061] Blocking: the PVDF membrane was blocked with 5% skimmed milk powder at room temperature for 2 hours, and washed 3 times with TBST buffer.
[0062] Incubation with primary antibody: BALB / c mouse serum infected with Schistosoma japonicum for 42 days, serum of New Zealand white rabbits infected with Schistosoma japonicum for 42 days, and serum of Schistosoma japonicum patients were added respectively, and mouse anti-His-Tag antibody (Abimate Biomedical Co., Ltd. company) as a positive control, healthy mouse serum as a negativ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com