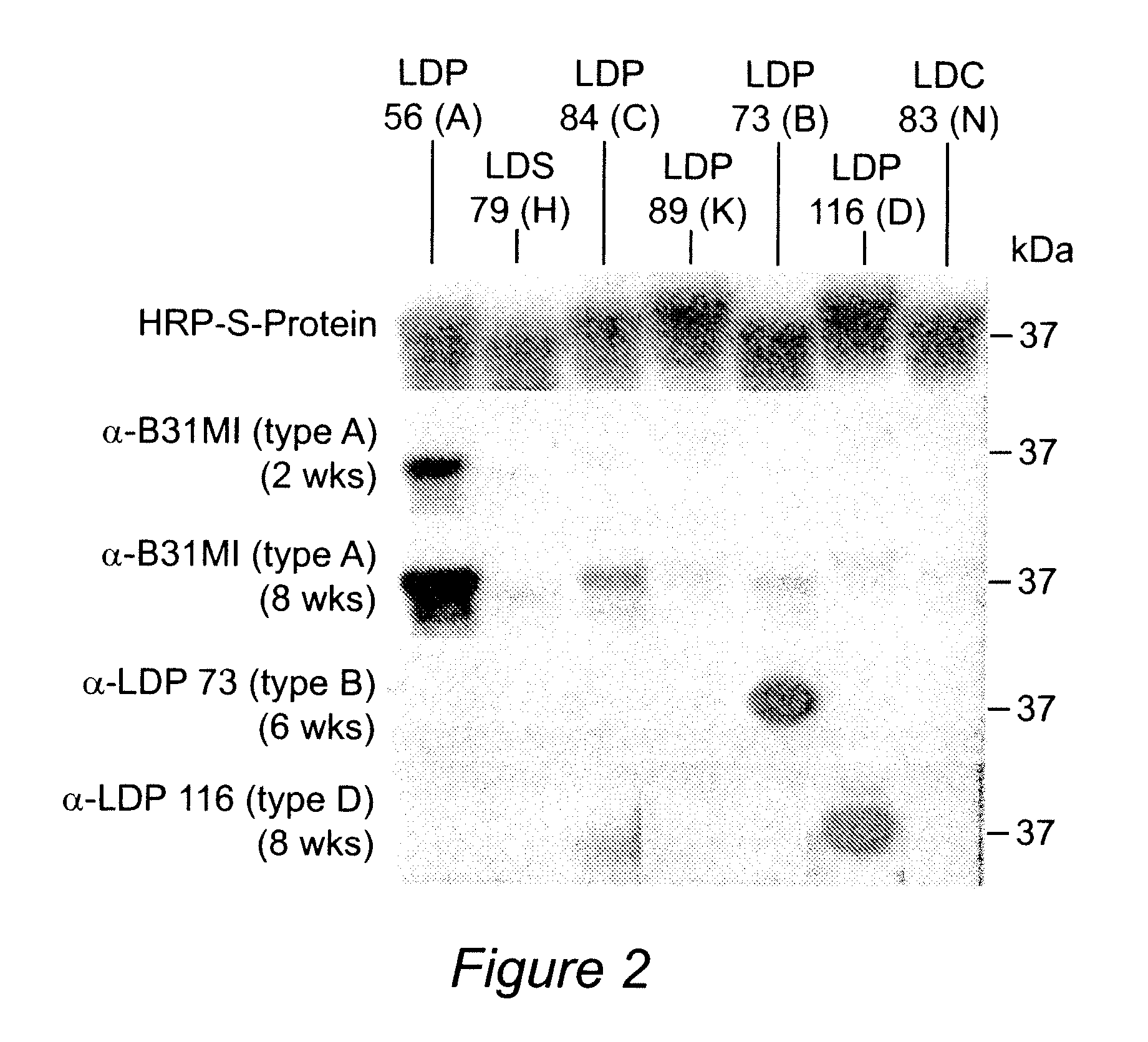
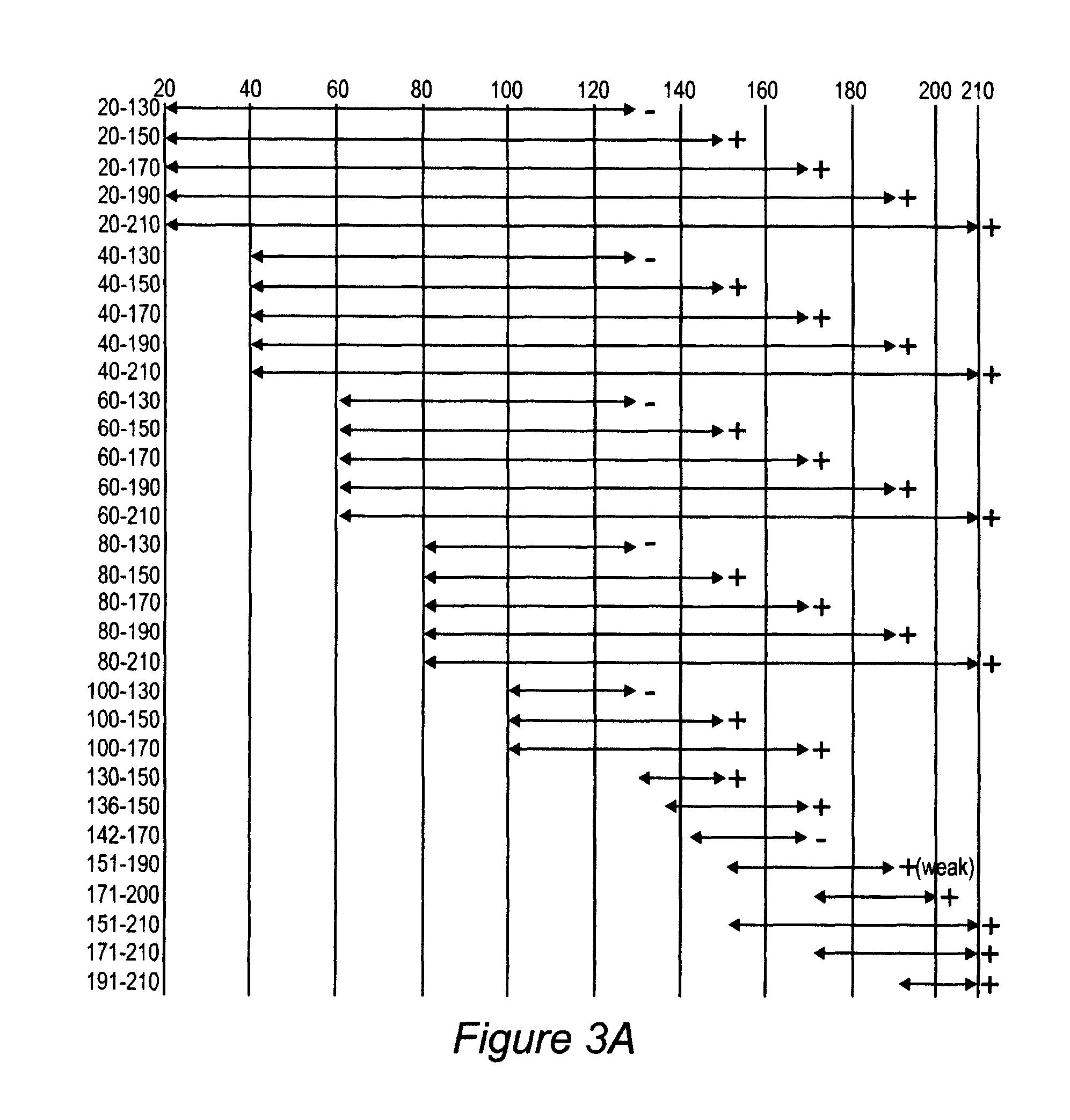
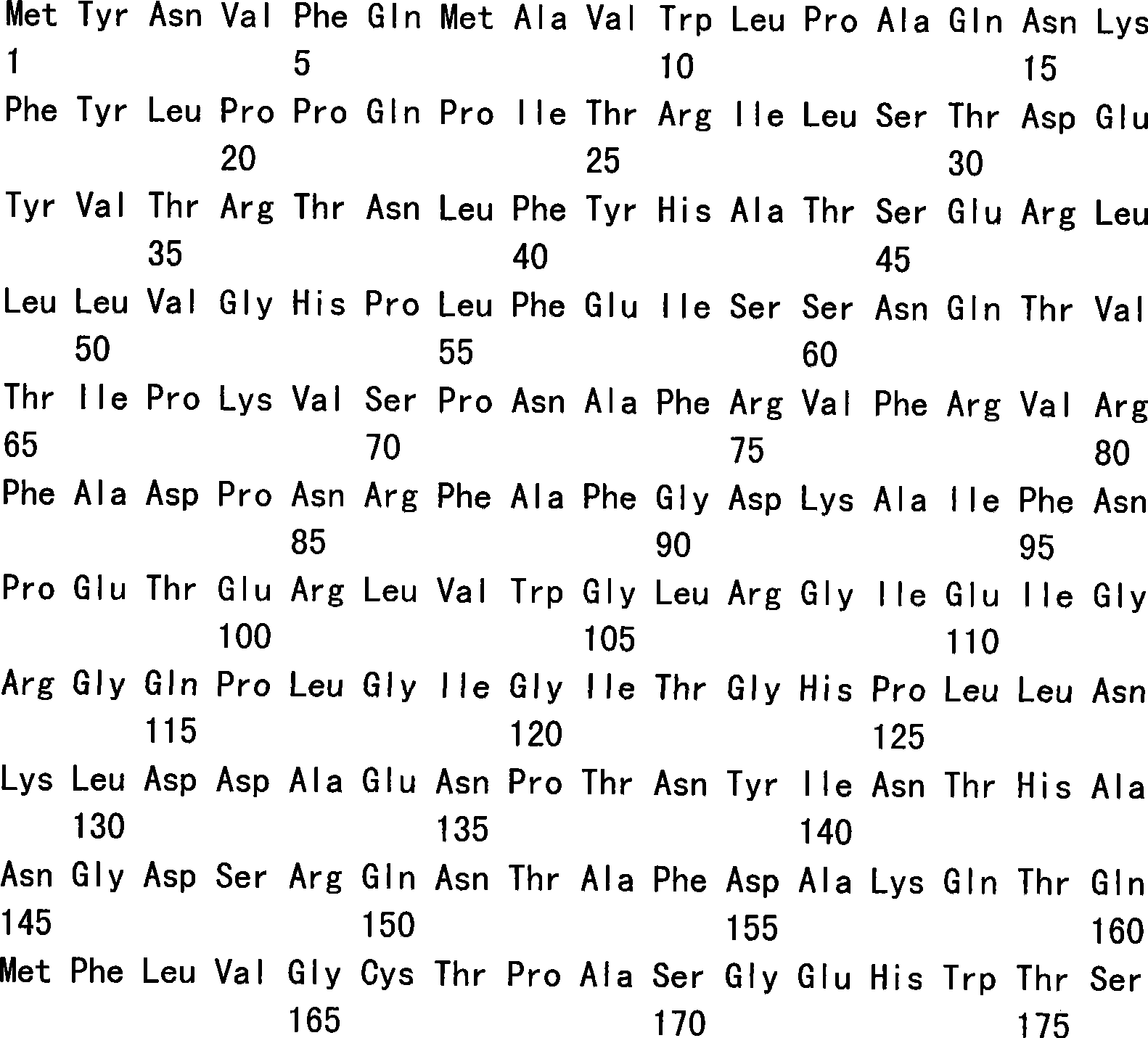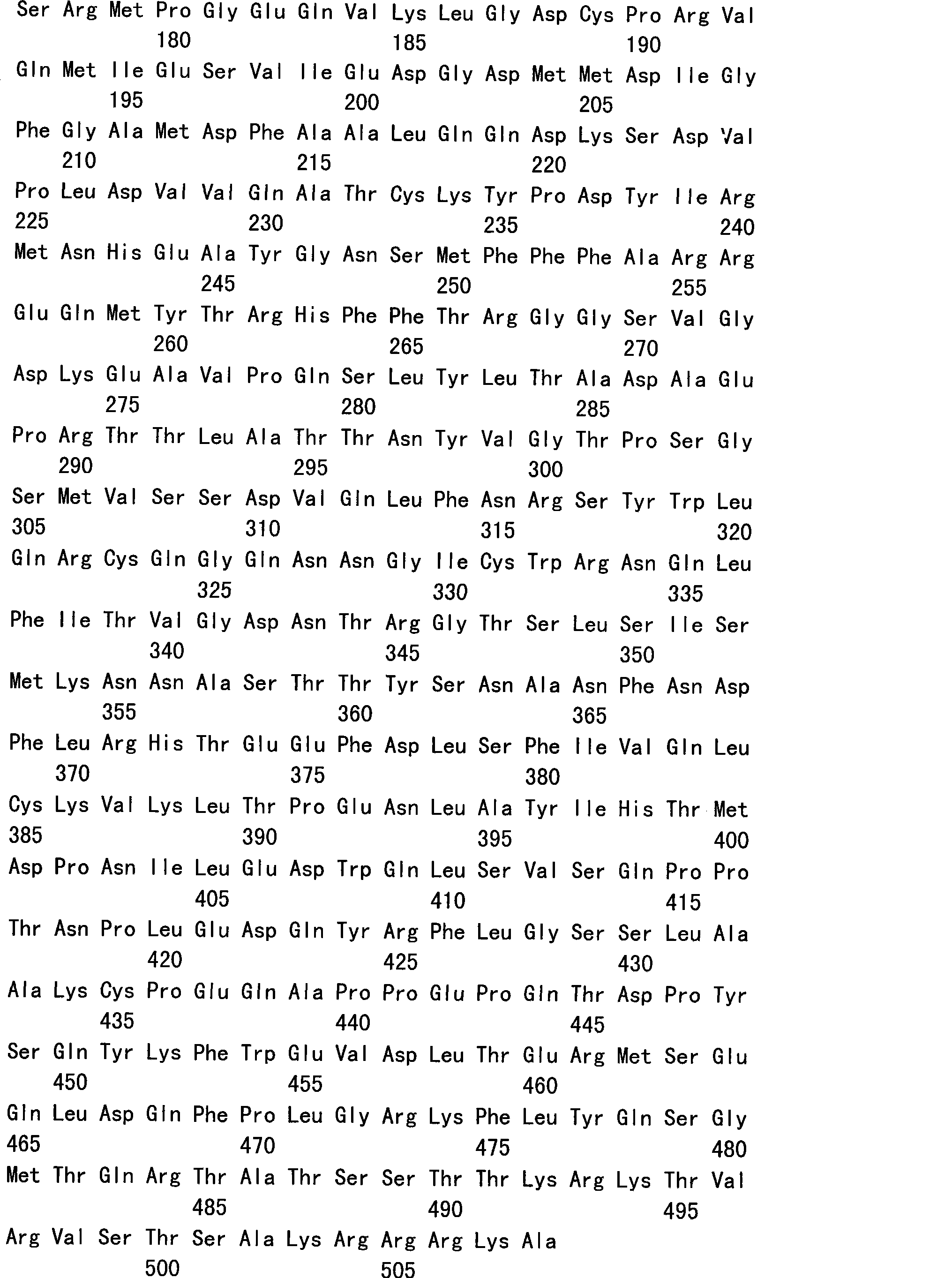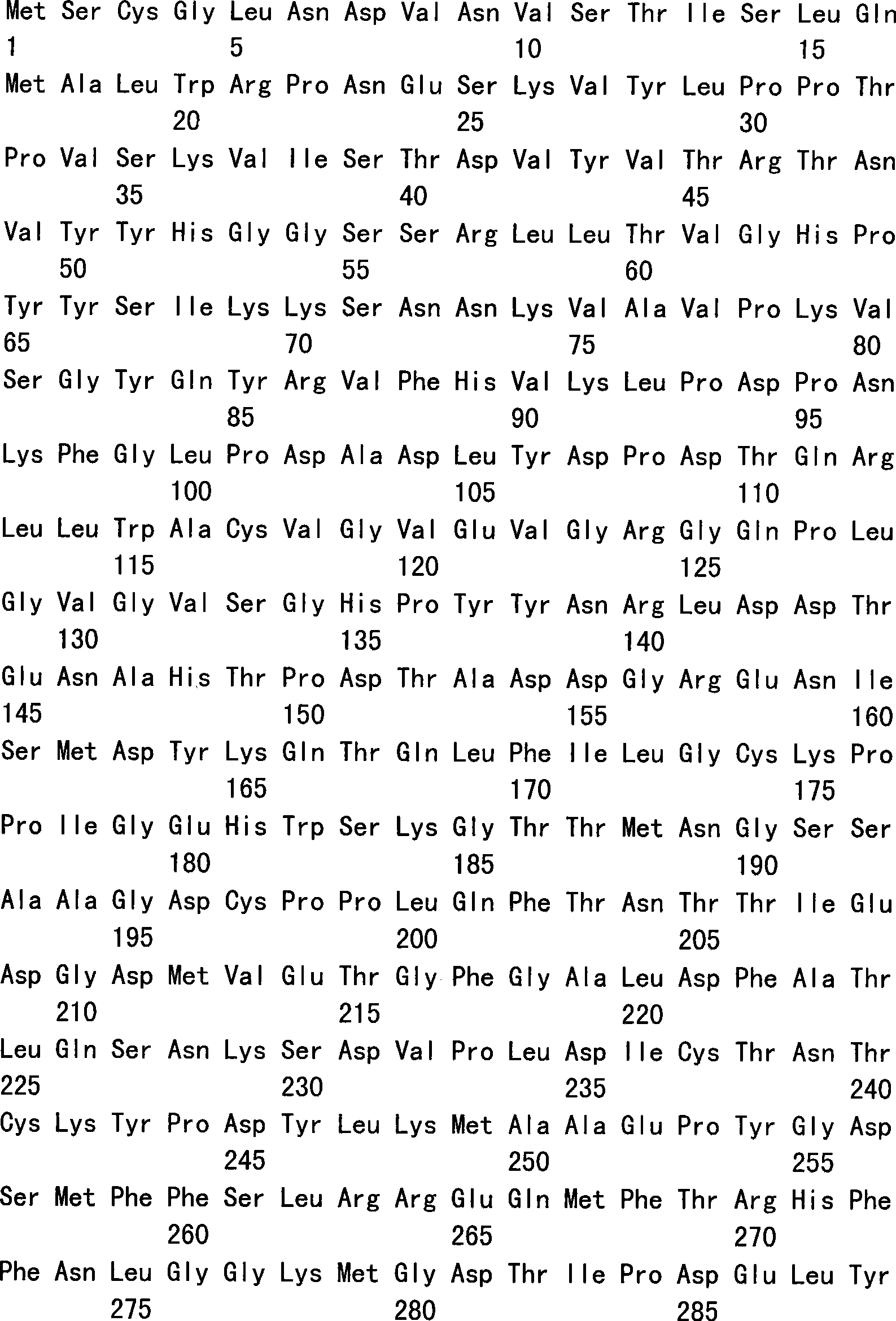Patents
Literature
121 results about "DIAGNOSTIC ANTIGENS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amino acid sequence of recombined human papilloma virus L1 capsid protein and uses thereof
InactiveCN101245099ABreak through the bottleneckControl the degree of aggregationBacteriaViral antigen ingredientsPentamerNucleotide
The invention relates to an amino acid sequence of a recombinant human papillomavirus L1 capsid protein, a nucleotide sequence which encodes the amino acid sequence and a recombinant vector and an expression host which contain the nucleotide sequence. The invention further relates to an application of a HPV L1 protein which is composed of the amino acid sequence in the preparation of vaccines, drug combination and diagnostic antigens or antibodies. The invention allows the recombinant HPV L1 capsid protein which is expressed in a prokaryotic system to be dissolvable in water by the modification of the HPV L1 wild-type sequence, and an L1 pentamer which has the same immunogenicity and antigenicity with the VLP of HPV L1 is obtained by expression. The invention allows the industrial production of the HPV L1 capsid protein by utilizing the prokaryotic expression system to become a reality, compared with the currently used eukaryotic expression system, the invention has the advantages of more stable quality of the products, higher yield, low cost and convenient quality control, which has great economic benefits and social effects.
Owner:马润林 +1
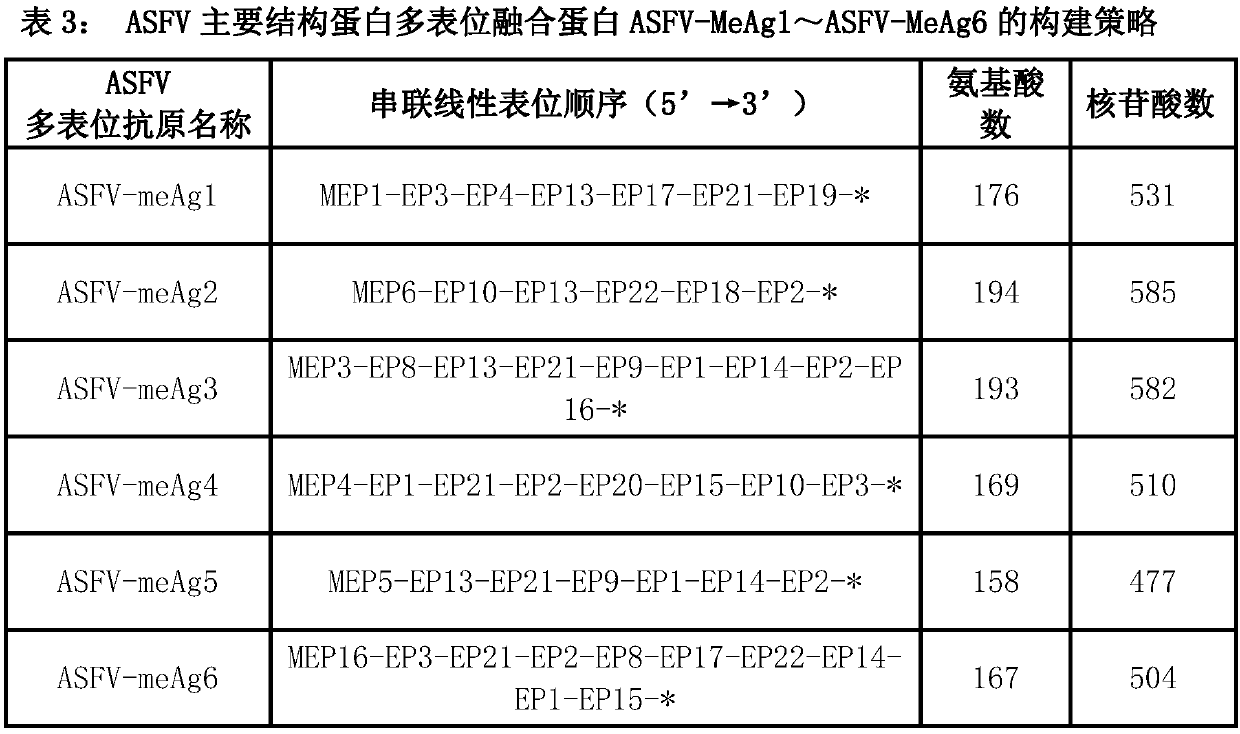
Multi-epitope fusion diagnosis antigen for African swine fever virus as well as preparation method and application thereof
InactiveCN108148138AImprove featuresIncreased sensitivityAntibody mimetics/scaffoldsVirus peptidesAntigenBacillus coli
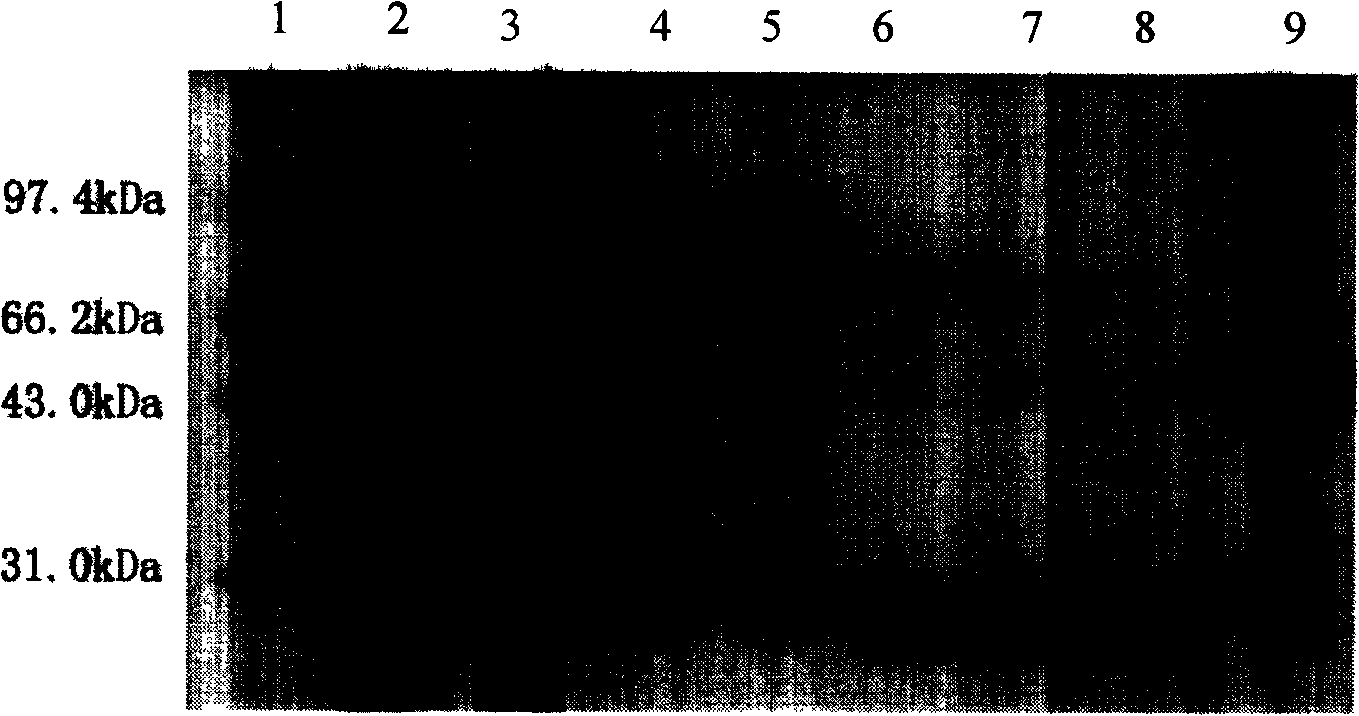
The invention discloses a multi-epitope fusion diagnosis antigen for African swine fever virus as well as a preparation method and application thereof. An ASFV (African swine fever virus) important structural protein gene encoding amino acid sequence is analyzed, screened and recombined through bioinformatics software, a multi-epitope fusion antigen gene is built and synthesized and is expressed in bacillus coli; through screening, the recombinant multi-epitope fusion antigen ASFV-meAg6 is obtained, so that diagnosis antigen protein with strong specificity and high sensitivity is provided foran ASFV serological diagnosis method.
Owner:SHIHEZI UNIVERSITY
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
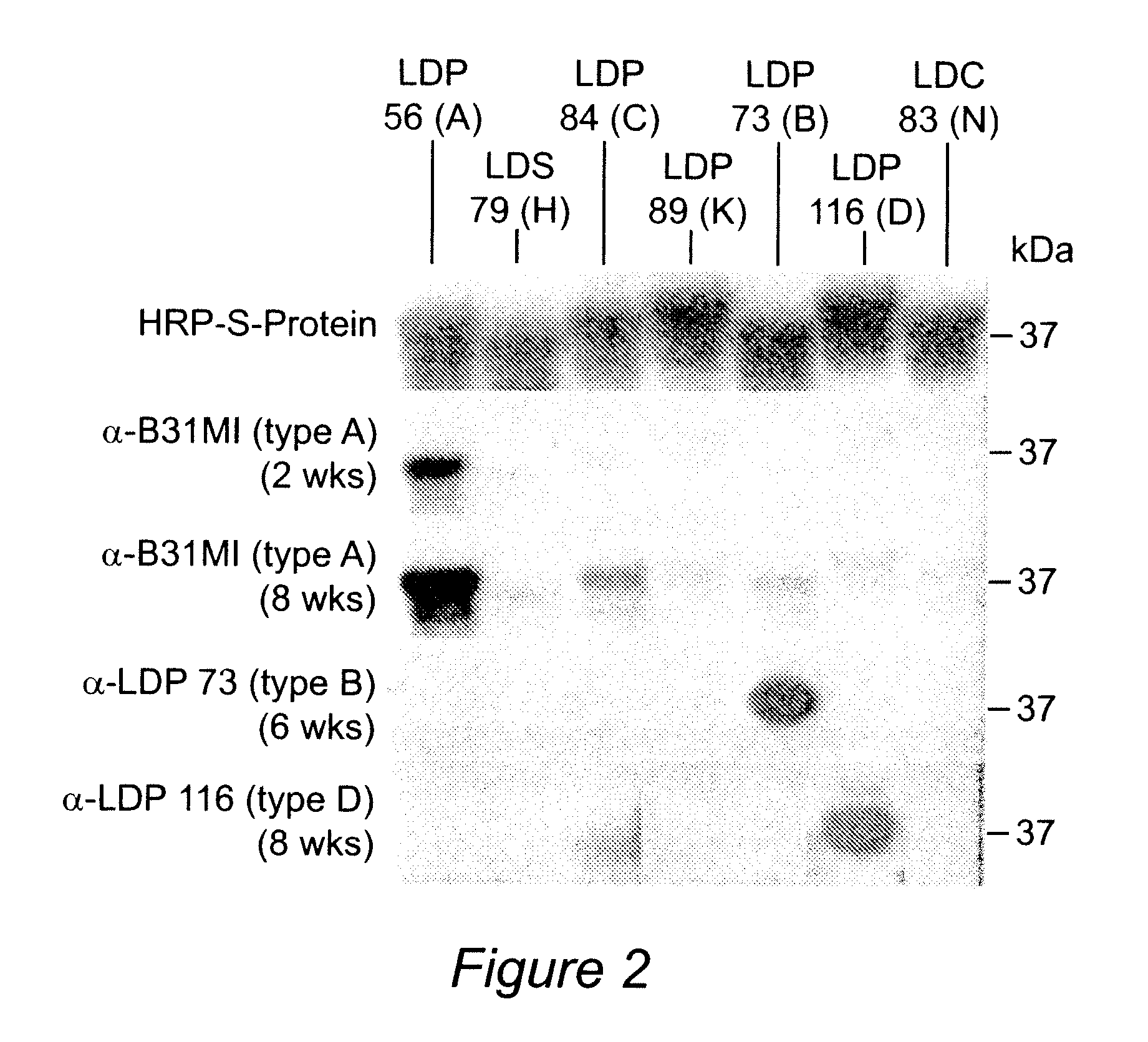
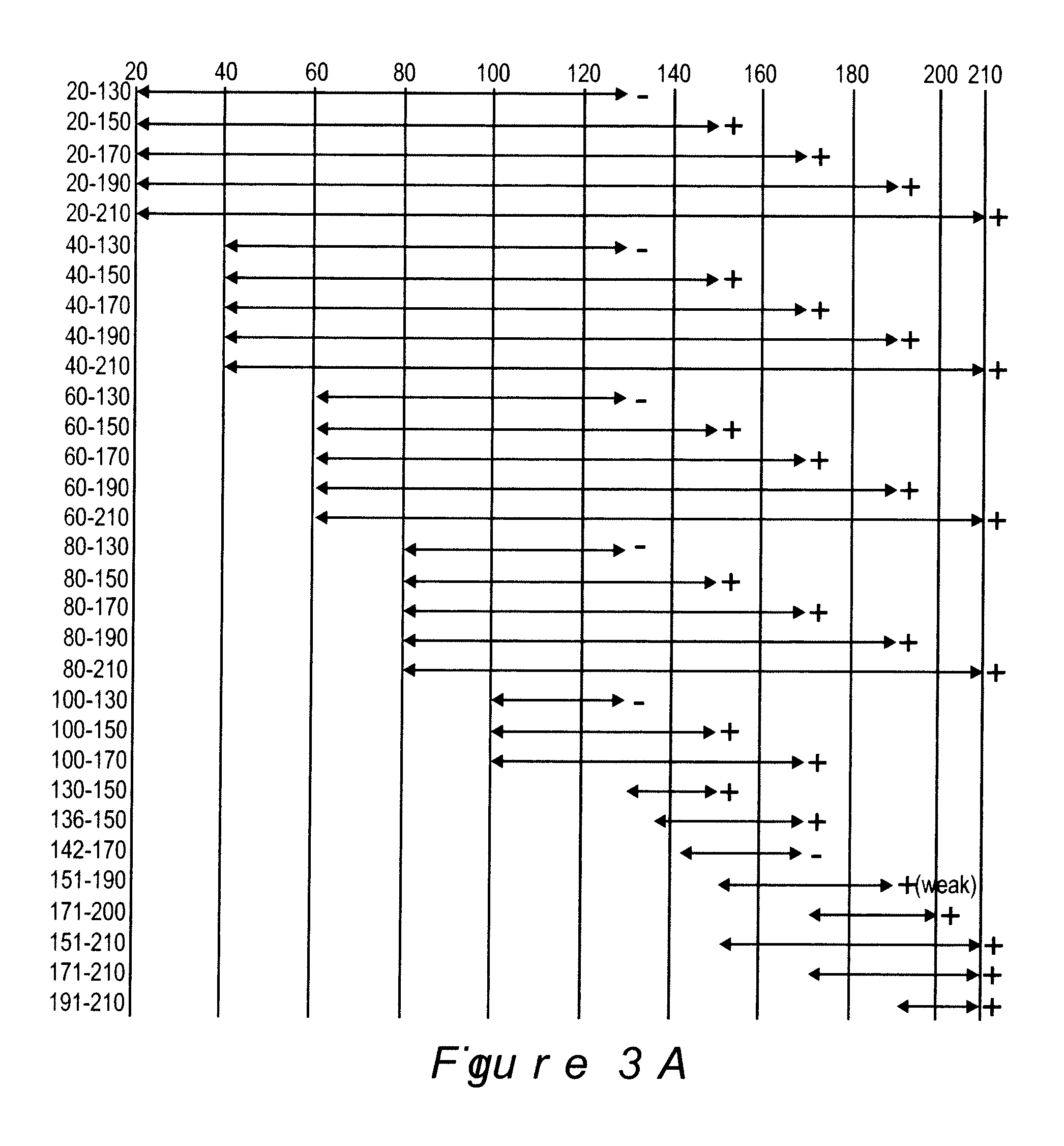
Polyvalent Chimeric OspC Vaccinogen and Diagnostic Antigen
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
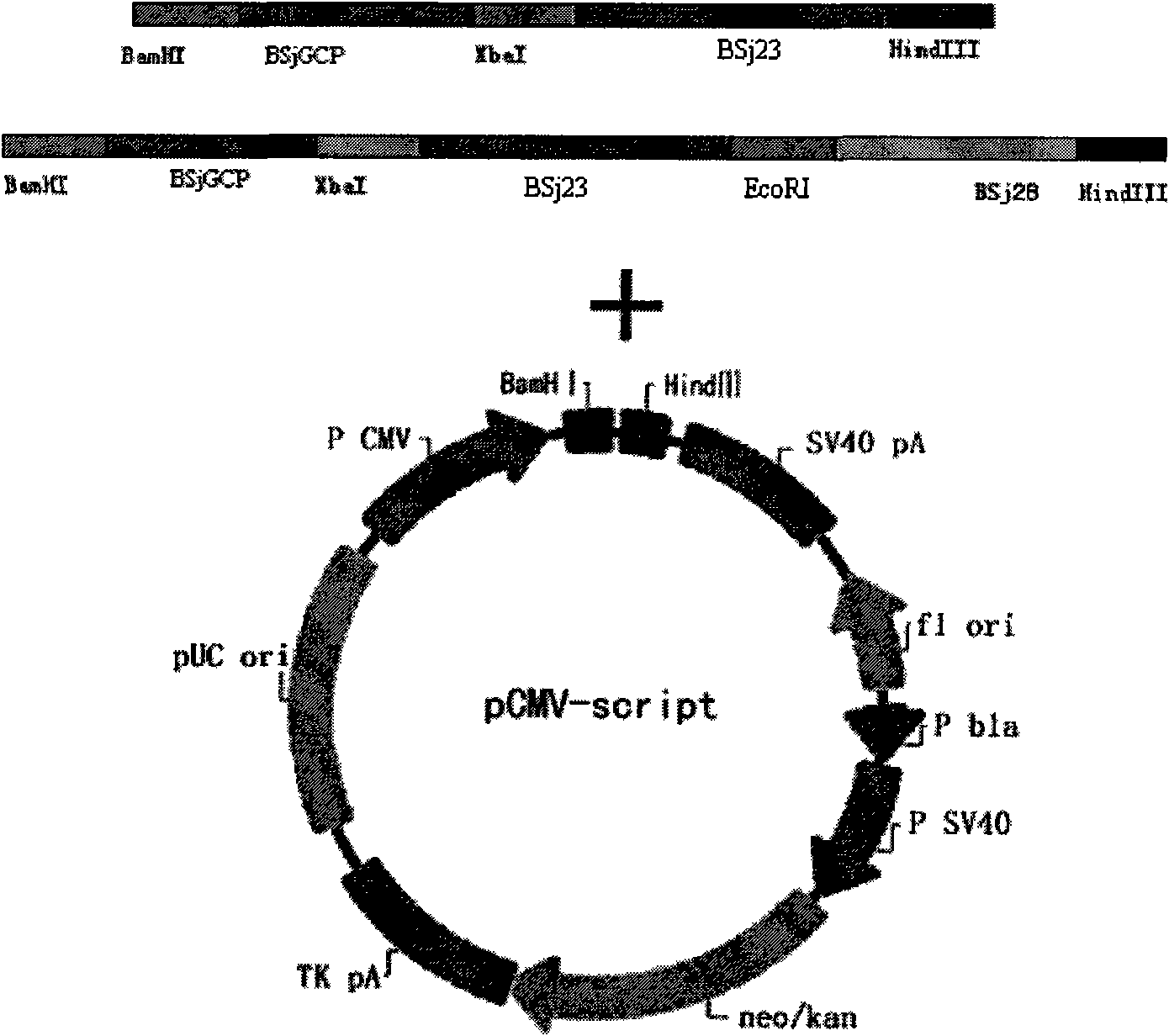
Schistosoma japonicum recombinant multi-epitope antigens, method for expressing and purifying same and application thereof
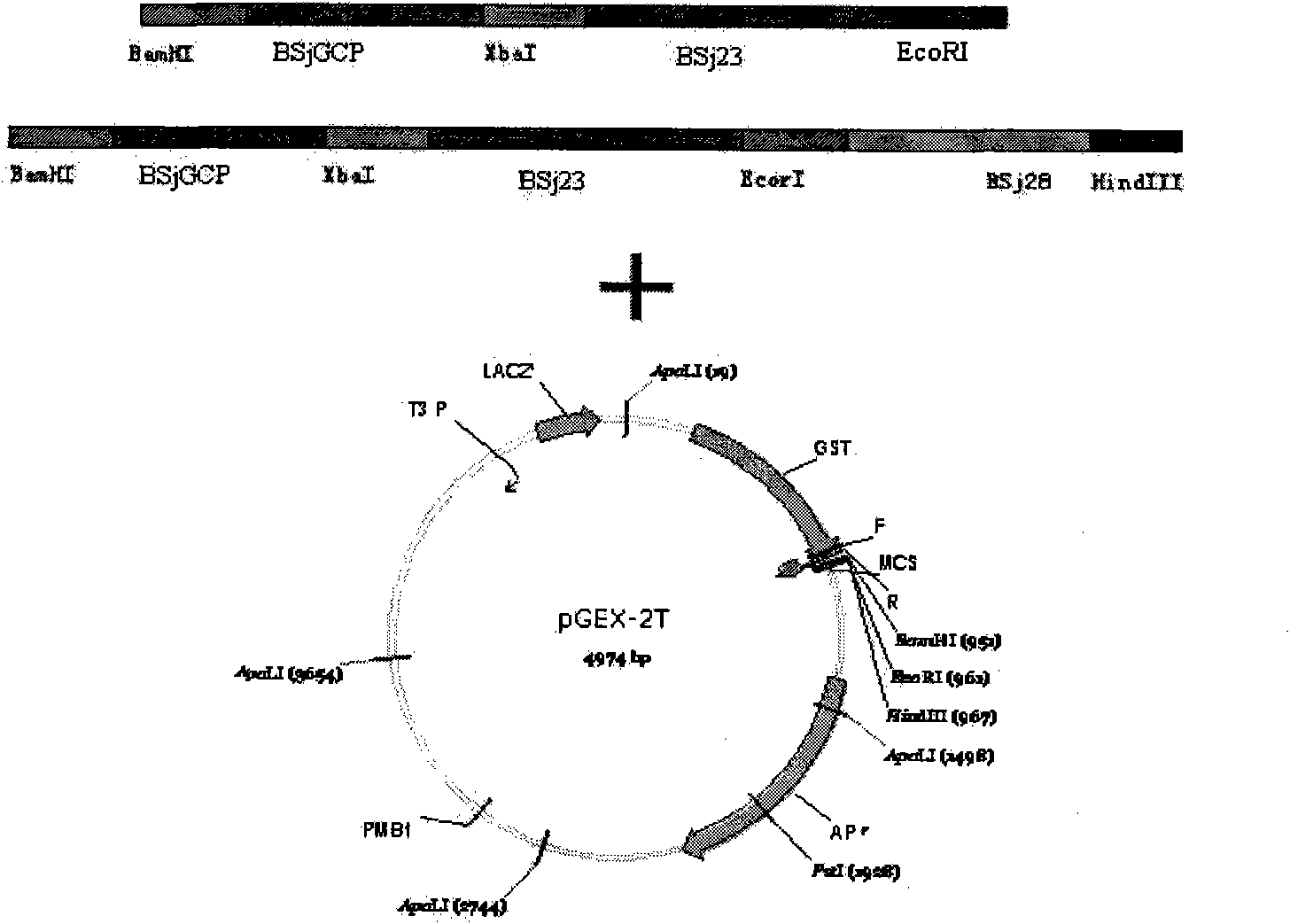
The invention discloses gene orders of schistosoma japonicum recombinant multi-epitope antigens BSjGCP-BSj23 and BSjGCP-BSj23-BSj28, a method for expressing and purifying the same, and application thereof in preparing schistosomiasis japonica immunity prevention vaccines and diagnostic reagents. Recombinant multi-epitope nucleic acid vaccines pCMV-BSjGCP-BSj23 and pCMV-BSjGCP-BSj23-BSj28 obtain 14.76 percent and 64.95 percent of worm reduction rates respectively in Kunming mice. The recombinant multi-epitope antigens pGEX-BSjGCP-BSj23 and pGEX-BSjGCP-BSj23-BSj28 obtain 15.7 percent and 57.99 percent of worm reduction rates in immunizing BalB / c mice, and obtain 91.0 percent and 89.9 percent of sensitivities as well as 97.8 percent and 93.4 percent of specificities respectively as diagnostic antigens.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Diagnostic reagent of tuberculosis and kit
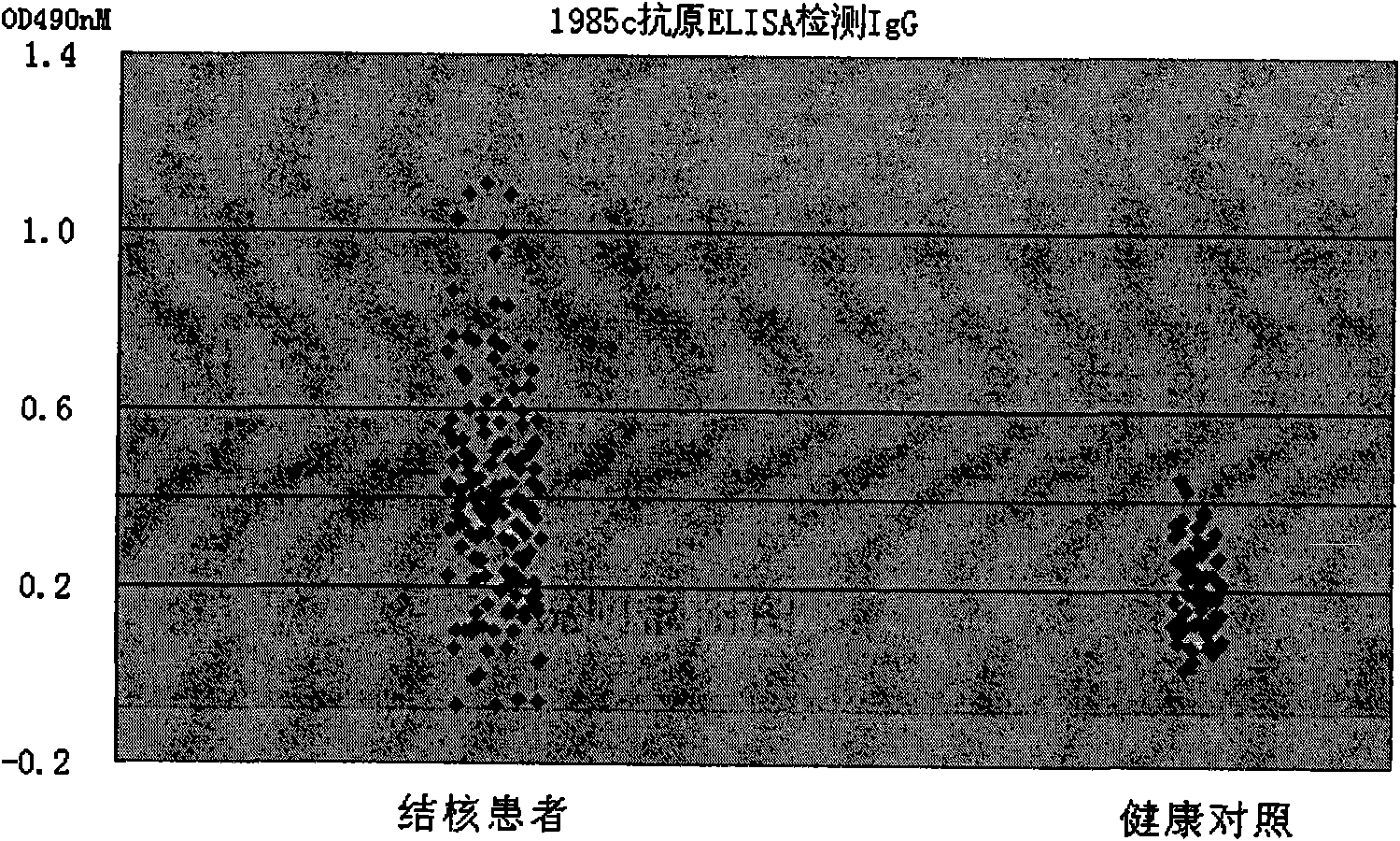
The invention belongs to the field of diagnostic reagents, and relates to a diagnostic reagent containing mycobacterium tuberculosis Rv1985c protein and a kit. The diagnostic reagent and the kit can perform rapid diagnosis on whether the blood or the body fluid of an experimenter is infected by the mycobacterium tuberculosis in the time periods from 15 minutes of an immune colloidal gold to 5 hours of ELISA. When the diagnostic reagent is used for the clinically-proved abortive tuberculosis, a Rv1985c antigen is used singly to detect that the sensitivity reaches 59 percent and the specificityreaches 96 percent; and when used together with other diagnostic antigens (such as a control antigen LAM / 38kDa), the diagnostic reagent can further improve the diagnostic sensitivity to 75 percent and has high clinical application values. The detection with the diagnostic reagent only needs a single blood serum sample, is simple and quick in test, needs no specialized laboratory equipment, is lowin cost, offers test results in the same day, and is very suitable for the detection of tuberculosis infection in extensive rural hospitals of villages and towns and under the condition of battlegrounds.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Latent human tuberculosis model, diagnostic antigens, and methods of use
Provided herein is an in vitro granuloma model and methods of its use. Methods of detecting and / or diagnosing latent tuberculosis in a subject are also provided, as are latency-specific antigens (and antibodies thereto), such as alpha-crystallin, and methods of identifying and using such molecules. Also provided are immunostimulatory compositions, for instance for use in eliciting an immune response in a subject, such as an immune response to a latent tuberculosis infection. Kits for carrying out the provided methods are also described.
Owner:DAVID S BEALL +3
Schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof
ActiveCN105384803AImproving immunogenicityGood antigenicityBiological material analysisAntiparasitic agentsDIAGNOSTIC ANTIGENSFhit gene
The invention provides a schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof. The protein has an amino acid sequence shown as SEQ ID NO.2 or an amino acid sequence having a same function, formed by replacing, omitting and / or adding one or more amino acid residues for the amino acid sequence shown as SEQ ID No.2. The invention also provides a gene sequence for encoding the protein. The recombinant protein SjSAPLP4 is good in immunogenicity, can be used as an excellent diagnostic antigen, can be used for preparing a schistosoma japonicum katsurada diagnosis kit having high sensitivity and high specificity, also can be used for preparing an anti-schistosome vaccine and can be used as a potential drug acting target spot to screen the drug for treating the schistosoma japonicum katsurada.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Enterovirus 71 type specific recombinant protein antigen and application thereof
InactiveCN102558313AIncreased sensitivityImprove featuresBacteriaMicroorganism based processesSerodiagnosesType specific
Owner:SOUTHEAST UNIV
Modification sequence of recombinant human mammilla tumor virus L1 capsid protein
InactiveCN101481407AStructural stability has no appreciable effectStructural Stability EffectsViral antigen ingredientsAntiviralsAntigenHuman papillomavirus
The invention relates to the prevention and treatment field of human papillomavirus infection, in particular to an amino acid modification sequence of recombinant human papillomavirus (HPV) L1 capsid protein, a nucleotide sequence encoding the amino acid modification sequence, and a carrier and a transformant containing the nucleotide sequence; and the invention further relates to application of an HPV L1 protein polymer consisting of the amino acid modification sequence to preparing vaccines, pharmaceutical compositions and diagnostic antigen or diagnostic antibodies. In the invention, an HPV L1 protein wild-type sequence is modified to prevent forming of a disulfide bond in the HPV L1 protein, the modification has no significant effect on the structural stability of the recombinant HPV L1 protein, but significantly improves the efficiency of purification process, and directly reduces the reagent consumption, thus effectively lowering the industrial production cost and having great economical benefit.
Owner:马润林 +1
Preparation of duck hepatitis I type virus indirect hemagglutination diagnostic antigen and kit
InactiveCN101423548AQuick responseEasy to useVirus peptidesMaterial analysisDuck hepatitis A virusType antigen
The invention relates to a method for preparing Duck hepatitis virus I type indirect blood coagulation diagnosing antigen, in particular to a method for preparing indirect blood coagulation diagnosing antigen by Duck hepatitis virus I type antigen sensibilization double hydroformylation mutton red cell. The Duck hepatitis virus I type indirect blood coagulation diagnosing antigen produced by the method has long storage time up to half a year; moreover, the diagnosing time is shortened and only half an hour is needed, and the use is convenient and simple; therefore, the method is suitable to be popularized and applied to the practical production.
Owner:陶海静
Preparation of Zika virus multi-segment fusion protein and IgG/IgM antibody detection kit
InactiveCN106841601AQuick checkDisease diagnosisAgainst vector-borne diseasesBiotin-streptavidin complexIgm antibody
The invention aims at providing a simple and quick Zika virus detection kit. The kit optimally selects fusion expression protein as diagnostic antigen; an anti-human IgG monoclonal antibody A374, an anti-human IgM monoclonal antibody A371 and a biotin-BSA conjugate are respectively coated on a nitrocellulose membrane as a detection line and a quality control line; colloidal gold labeled fusion expression protein and colloidal gold labeled streptavidin and other reagents are matched; and an immunochromatography capture method principle is used for qualitative detection of Zika virus specific IgM antibody and IgG antibody in human serum, thereby realizing quick and specific diagnosis of Zika virus infection.
Owner:GUANGZHOU DARUI BIOTECH
Polyvalent chimeric OspC vaccinogen and diagnostic antigen
InactiveUS7794727B2Broad protectionAntibacterial agentsBacterial antigen ingredientsEpitopeAlpha helix
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
Theileria equi immunoblotting detecting method and method for preparing kit
The invention discloses a theileria equi immunoblotting detecting method and a method for preparing a kit, wherein the theileria equi immunoblotting detecting method in the invention is established on the basis of acquiring diagnostic antigen and positive control serum via Cochlearia officinalis hyperoxide enzyme label and chemical light emitting prime. The solvent for the method in the inventionis little in amount, low in cost, high in specificity, high in sensitivity, high and fast in operation, free of toxicity and harm, low in required conditions, easy for judging the test result, long-time for storage, and stronger in specificity than ELISA. The method in the invention detects no false positive and false negative result.
Owner:中华人民共和国天津出入境检验检疫局
Foot-and-mouth disease virus 2C3ABC recombinant protein as well as preparation method and application thereof
InactiveCN104788547AShow detection specificityShorten the emergency response time for prevention and controlSsRNA viruses positive-senseVirus peptidesBacteroidesSolubility
The invention discloses a foot-and-mouth disease virus (FMDV) 2C3ABC recombinant protein as well as a preparation method and application thereof, and belongs to the field of pharmaceutical biotechnology. According to the invention, the mutation of the following sites is performed on the basis of an original FMDV 3C protease gene: Cys142-Ser, Cys163-Gly. FMDV recombinant protein 2C3ABC is expressed as an inclusion body in the bacterial cytoplasm, and is subjected to separation, denaturation, renaturation and multi-step purification, to obtain a complete and enzymolysis-free FMDV nonstructural protein mu2C3ABC, wherein the protein has solubility and complete antigenicity, and has a molecular weight of 72 kDa. The protein, as a diagnostic antigen, is prepared into chromatographic strips, has sensitivities of 98.4% and 100% respectively in the detection of FMDV experimentally infected pigs and naturally infection-free pigs, and has specificities of 100% and 98% respectively in the detection of naturally infection-free pigs and vaccine-immunized pigs. Compared with commercial kits Ceditest and UBI, the FMDV 2C3ABC recombinant protein has a high degree of consistency, can be used to distinguish infected animals and immunized animals, wherein K = 0.823 (p is smaller than 0.05).
Owner:吕宏亮 +2
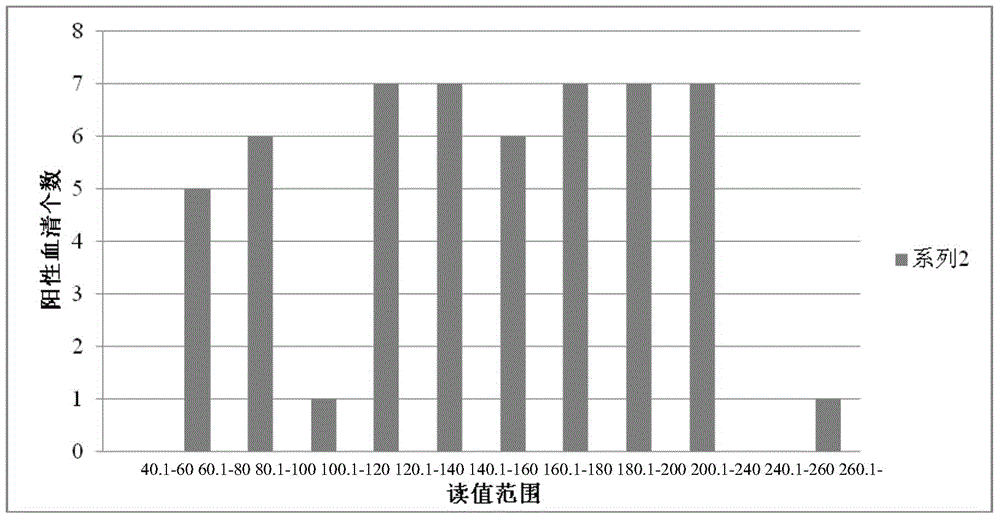
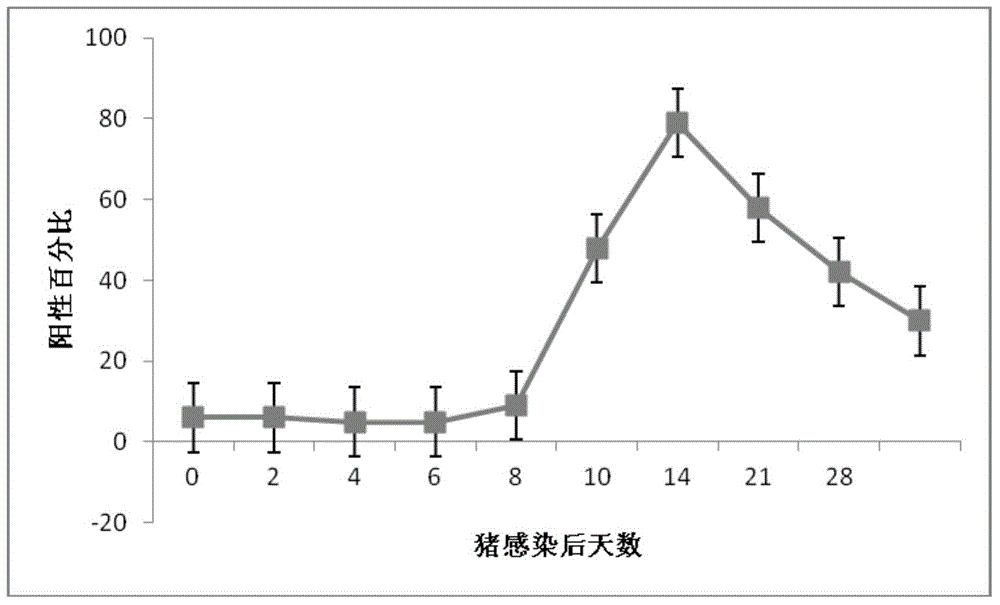
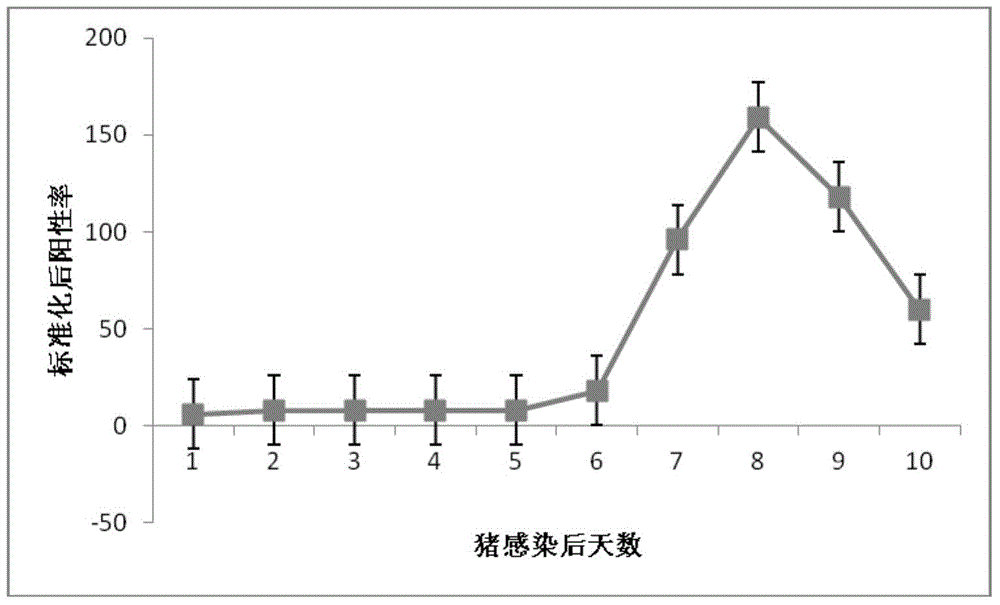
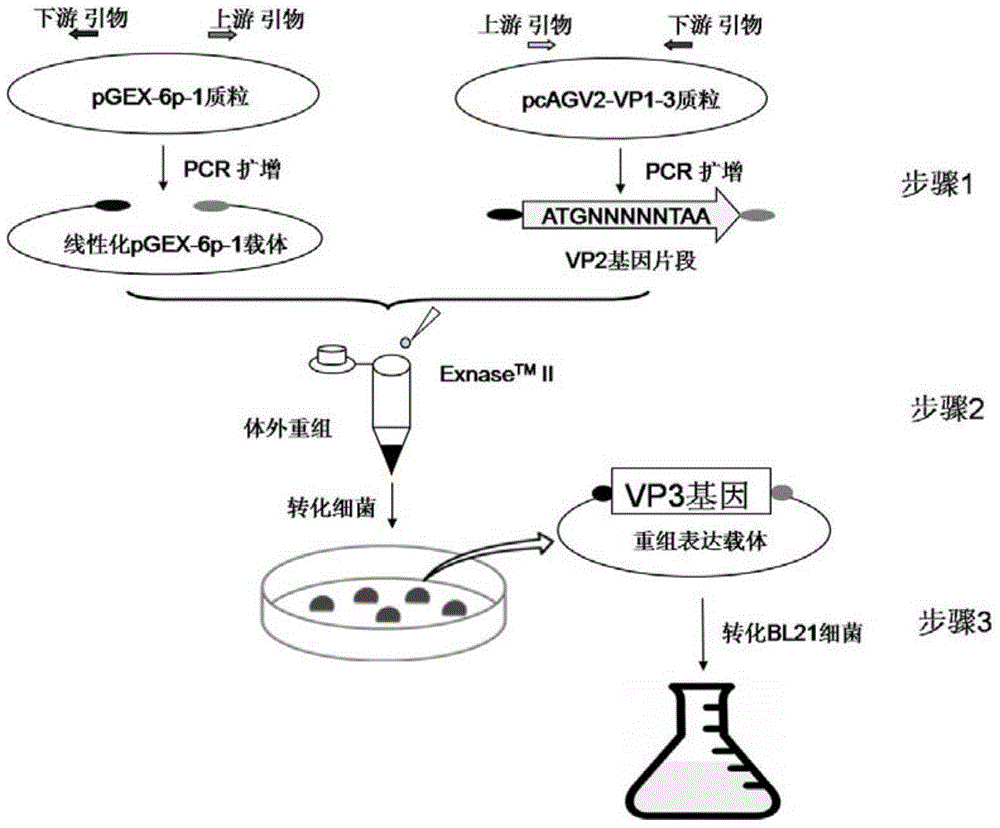
AGV2 (avian gyrovirus 2) type soluble VP3 (viral protein 3) and preparation method thereof
InactiveCN105037503ARapid clone expressionSimplify the cloning processVirus peptidesMicroorganism based processesEscherichia coliEnzyme digestion
The invention discloses an AGV2 (avian gyrovirus 2) type soluble VP3 (viral protein 2) and a preparation method thereof. The preparation method comprises the following steps: using a pGEX-6p-1 linearization carrier and a primer of an AGV2 VP3 gene segment; directly and quickly recombining and cloning VP3 in vitro by using recombinase ExnaseTM II without carrying out enzyme digestion ligation reaction; converting escherichia coli; carrying out inducible expression through IPTG, so as to achieve the fusion soluble expression of VP3 of AGV2 and GST and obtain purified soluble VP3. The AGV2 VP3 soluble expression and purified protein obtained through the preparation method can directly provide the soluble VP3 as an AGV2 diagnostic antigen and as an immunogen to obtain an anti-VP3 polyclonal antibody, provides an effective immunology reagent for carrying out AGV2 serology epidemiology researches and VP3 functional study, fills up the blank at home and abroad, and has an important meaning for further exploring VP3 biological function.
Owner:YANGZHOU UNIV
Latent human tuberculosis model, diagnostic antigens, and methods of use
Provided herein is an in vitro granuloma model and methods of its use. Methods of detecting and / or diagnosing latent tuberculosis in a subject are also provided, as are latency-specific antigens (and antibodies thereto), such as α-crystallin, and methods of identifying and using such molecules. Also provided are immunostimulatory compositions, for instance for use in eliciting an immune response in a subject, such as an immune response to a latent tuberculosis infection. Kits for carrying out the provided methods are also described.
Owner:DAVID S BEALL +3
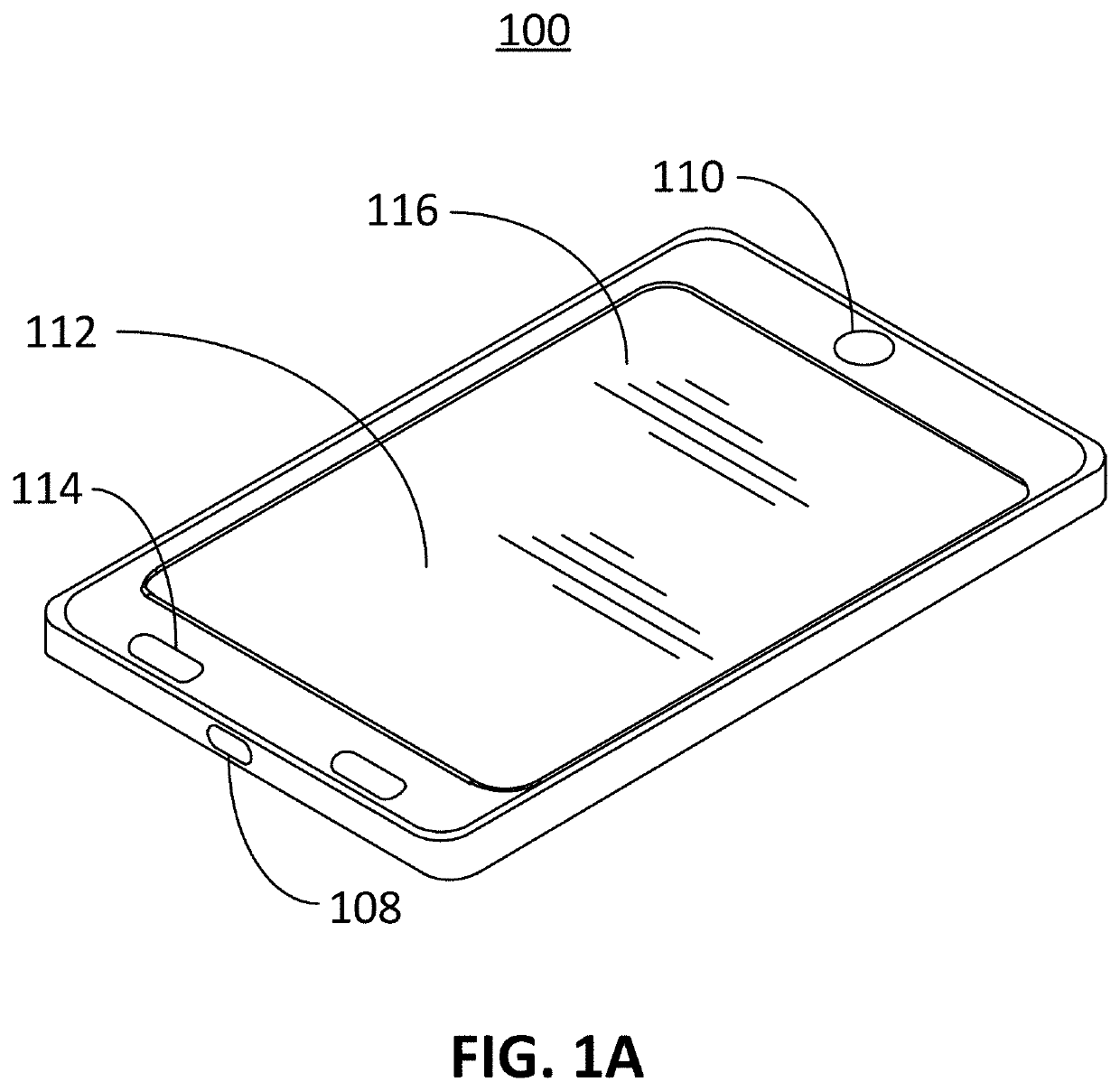
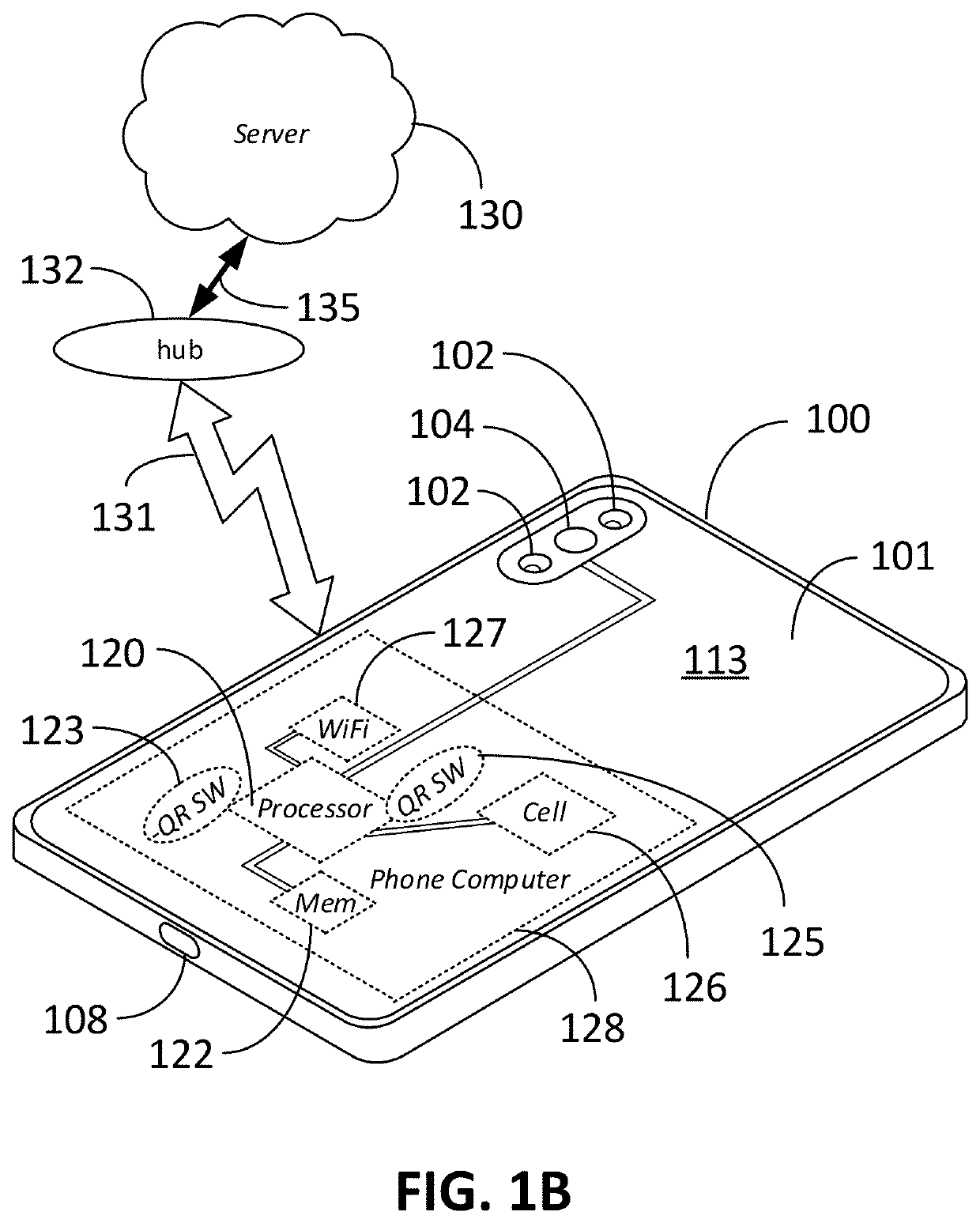
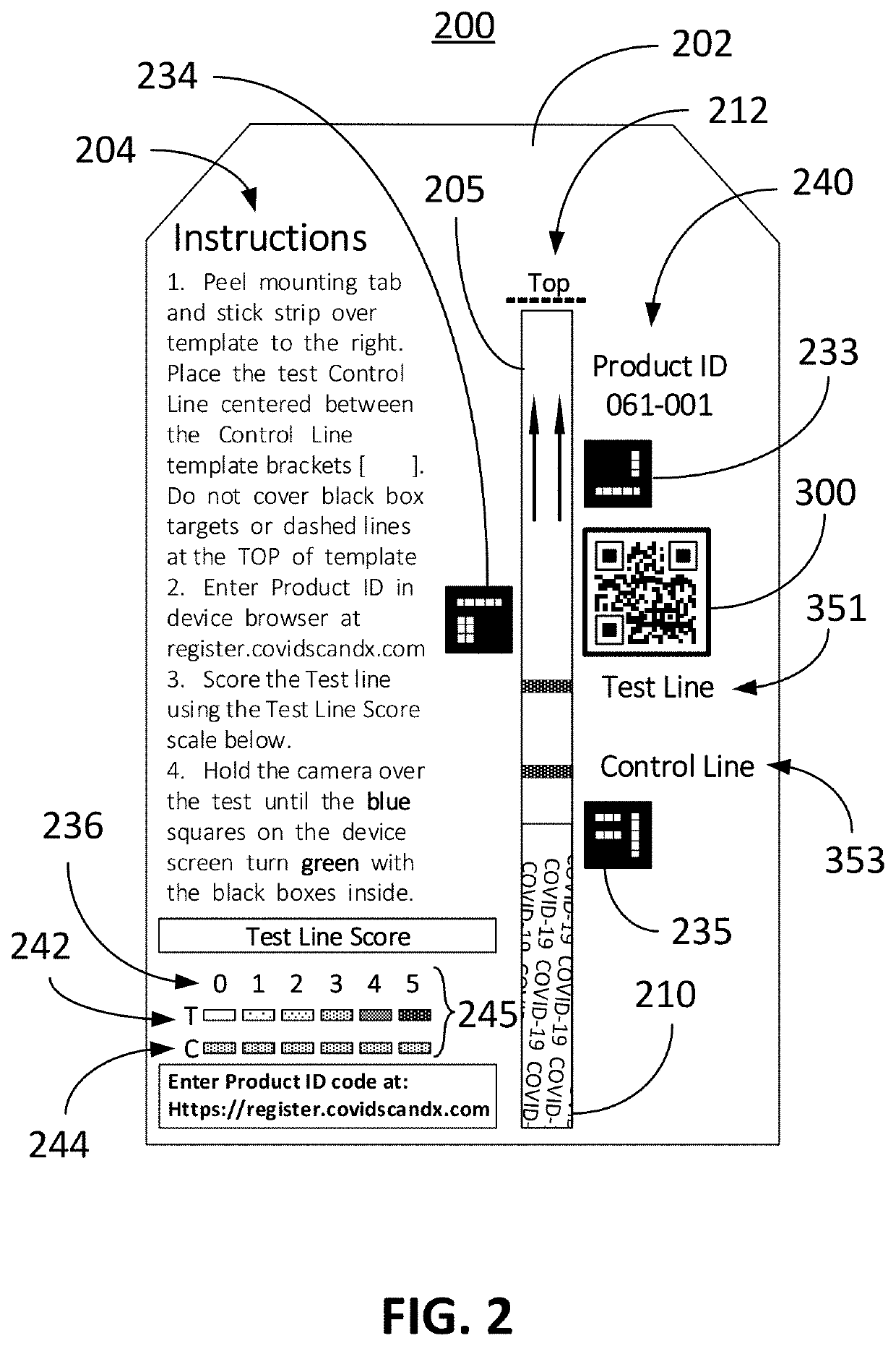
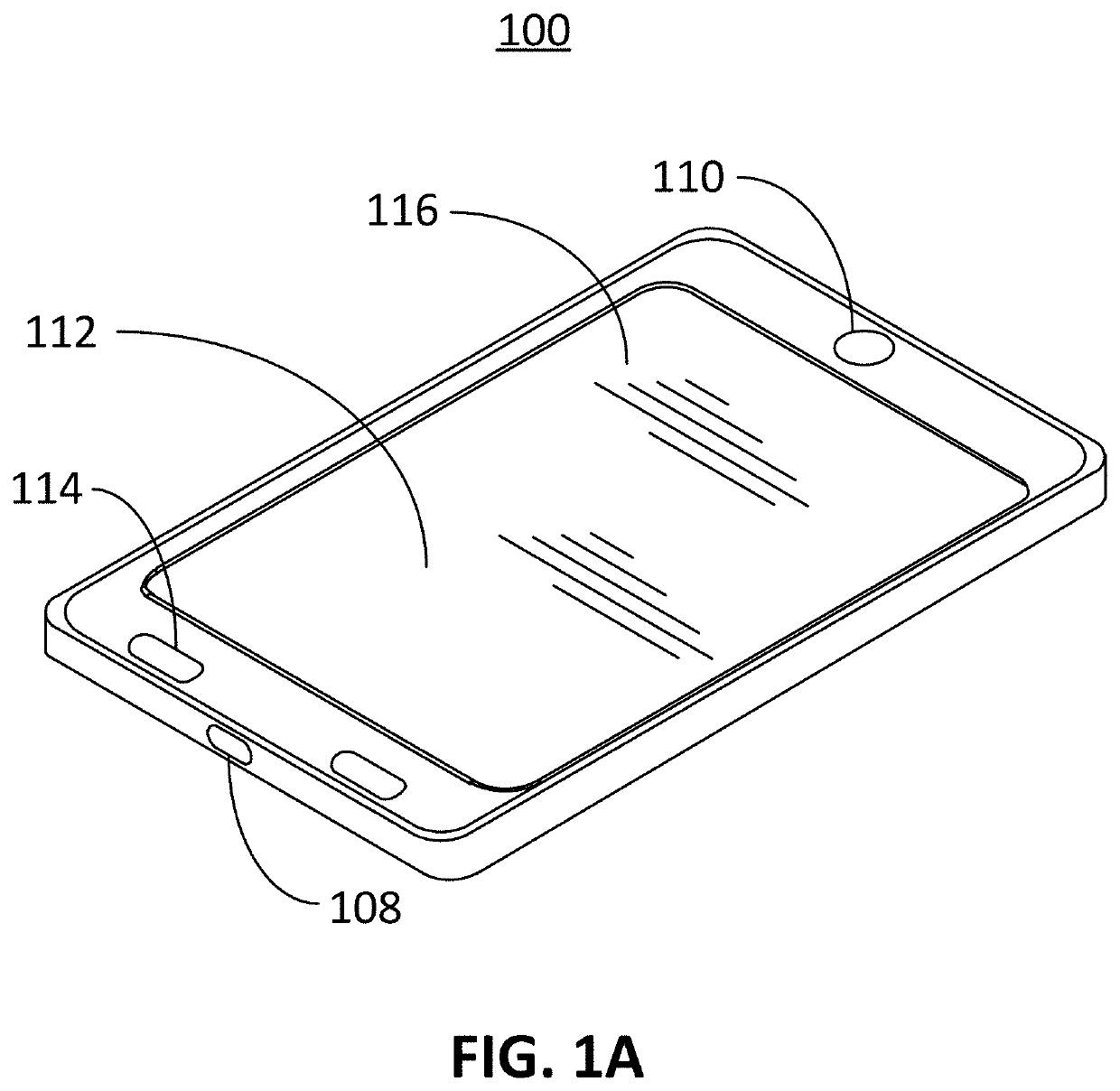
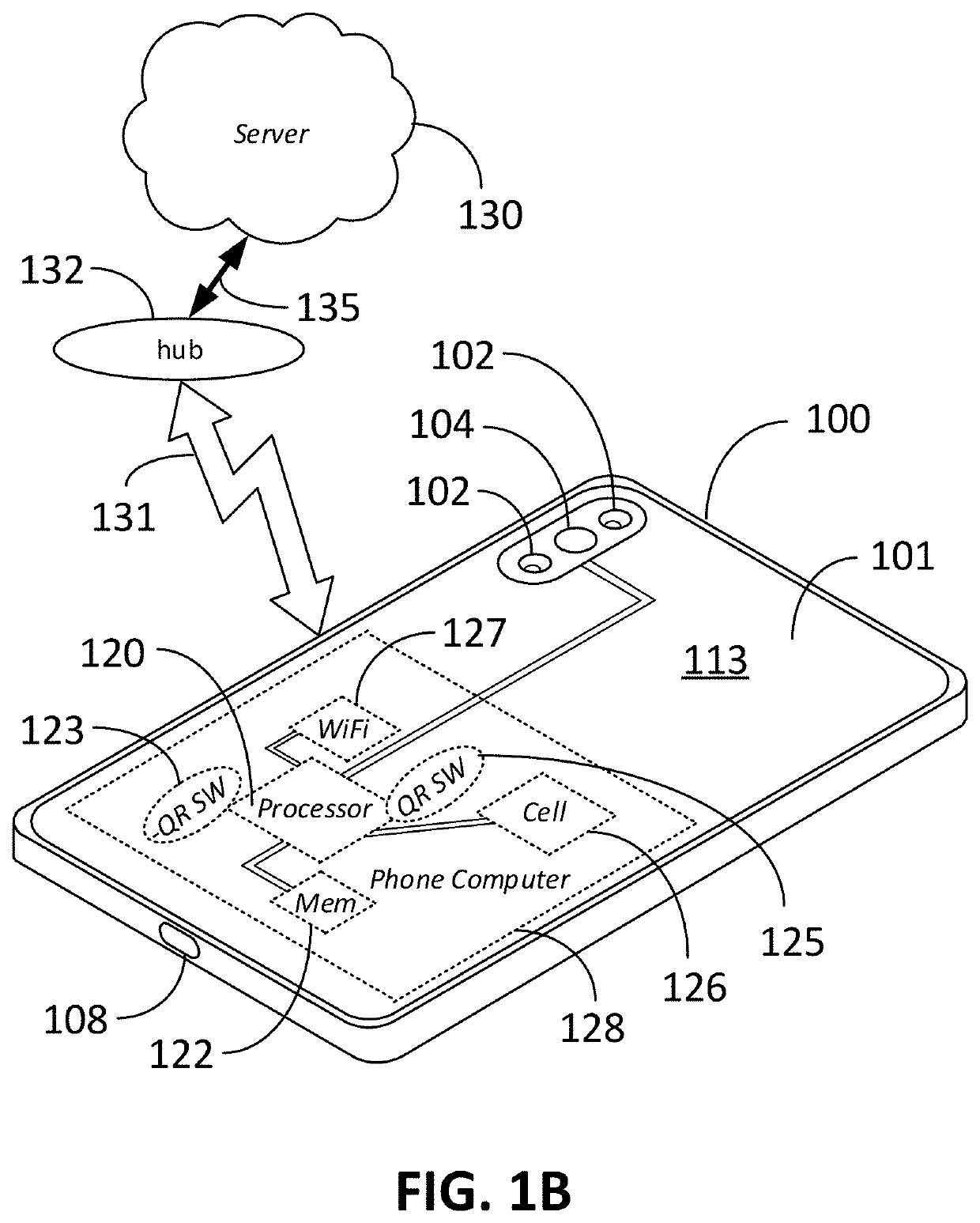
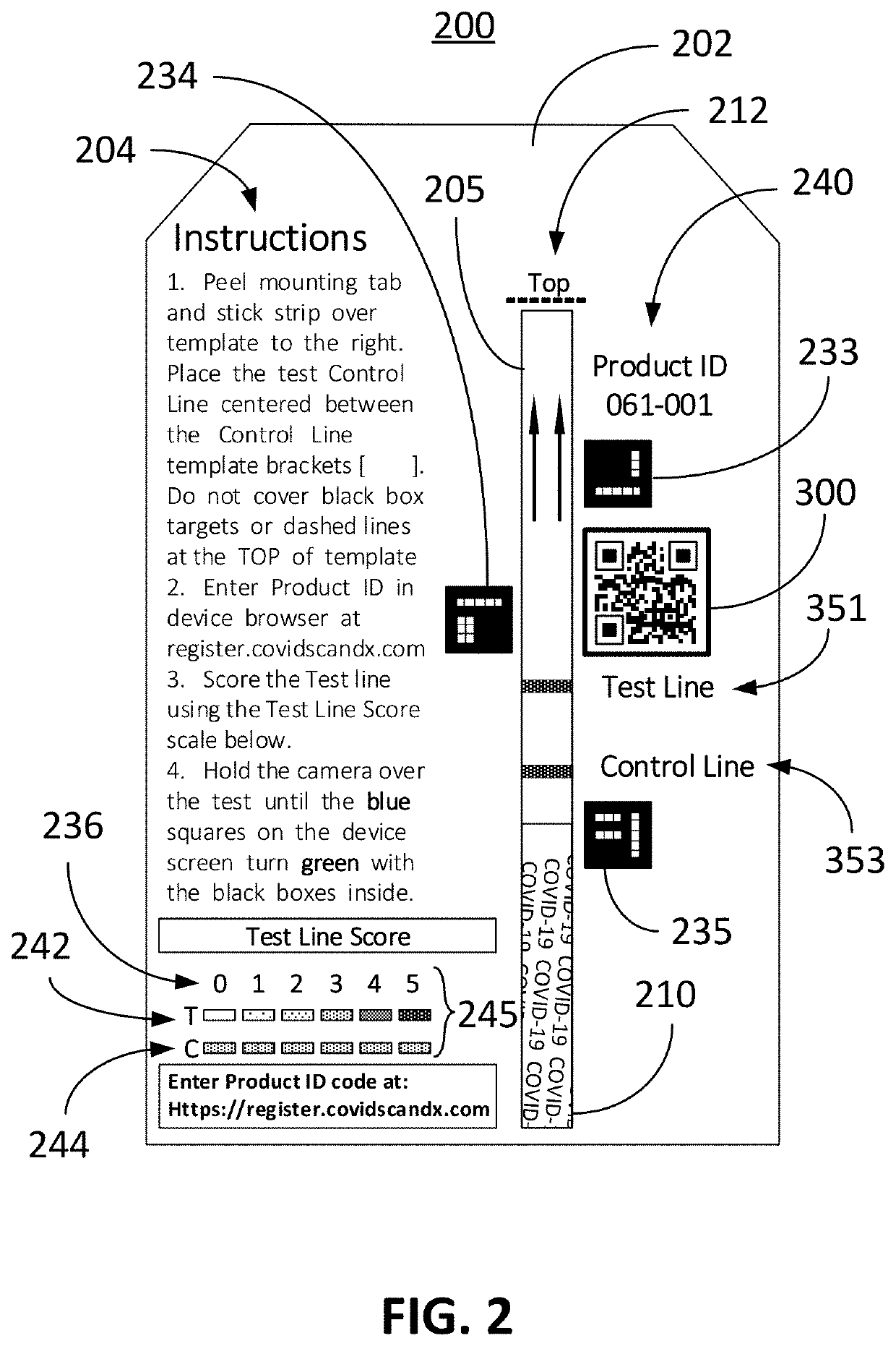
Computer vision method for improved automated image capture and analysis of rapid diagnostic test devices
ActiveUS20220027587A1Low efficacyImprove accuracyHealth-index calculationCharacter and pattern recognitionRapid screening testAntigen
The disclosed embodiments are generally directed to improving feature detection of rapidly acquired images using camera-enabled mobile devices involving a 2-D decal code, such as a QR code, for improving the reading accuracy of a rapid diagnostic antigen or antibody or enzymatic colorimetric directed test, such as for COVID-19 diagnosis. One primary issue with evaluating a Covid-19 rapid test is detecting and quantifying positive test lines from sampled test strips based on digital images of the test strip. Aspects of the present invention contemplate masking a QR code to improve the sample image resolution and contrast. Other aspects of the present invention contemplate methods and techniques to evaluate a test line on the sample image by enhancing an intensity curve along the test line and control line containing area by way of calculating the instantaneous change in pixel intensity and evaluating the position and intensity of those signals.
Owner:NEUROGANICS DIAGNOSTICS LLC
Recombinant alpha protein for inhibiting clostridium perfringens infection and preparation method and application thereof
InactiveCN106008684AHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsSolubilityProtein tag
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
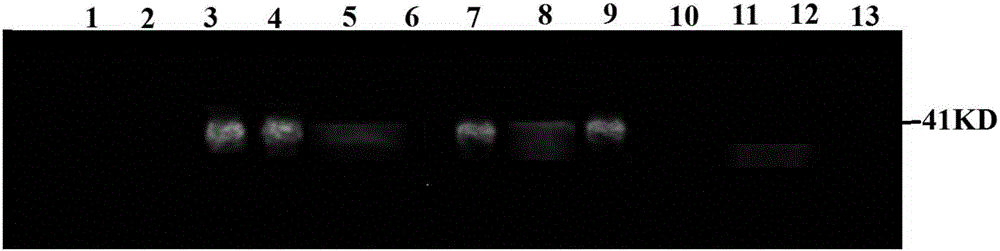
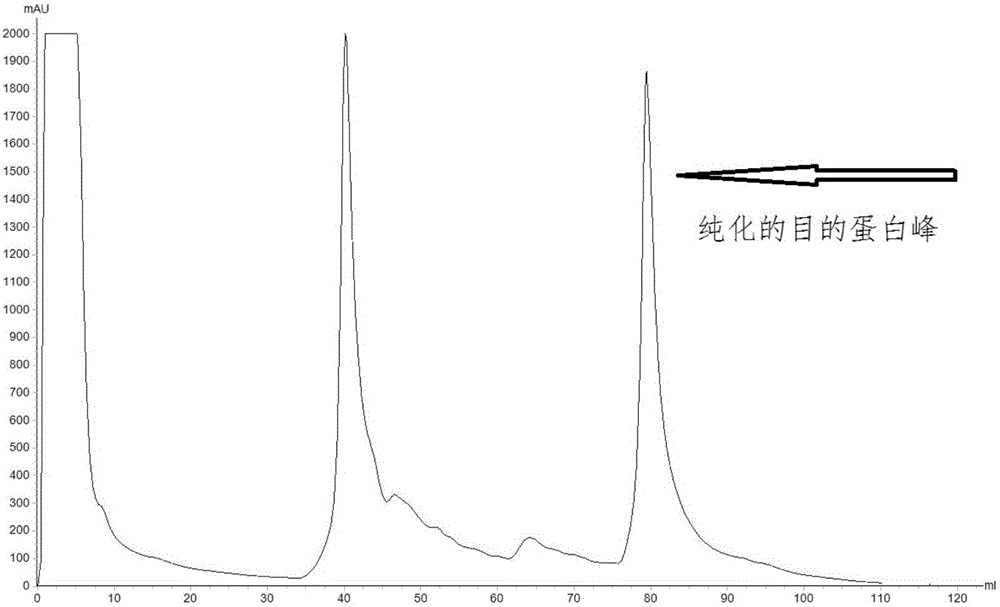
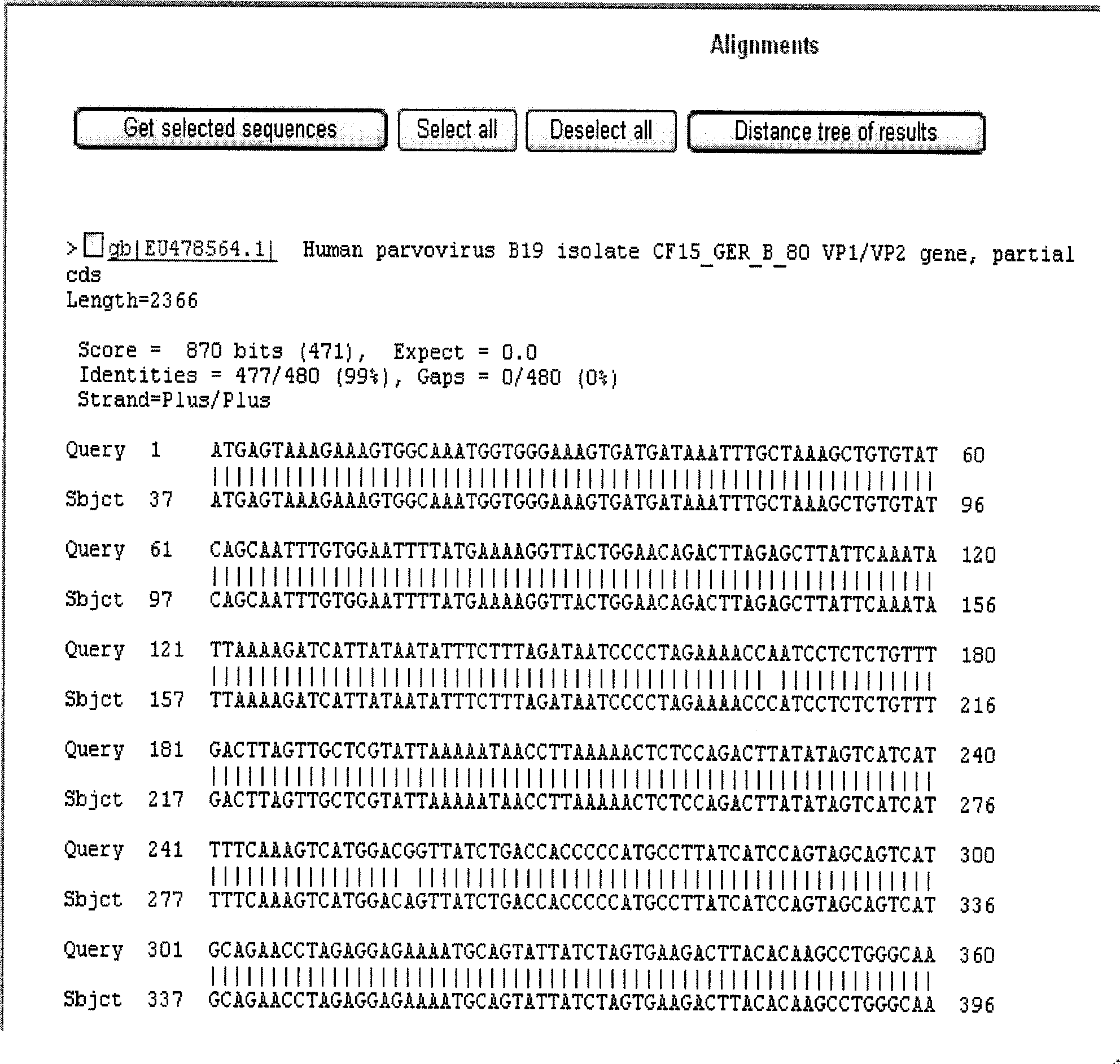
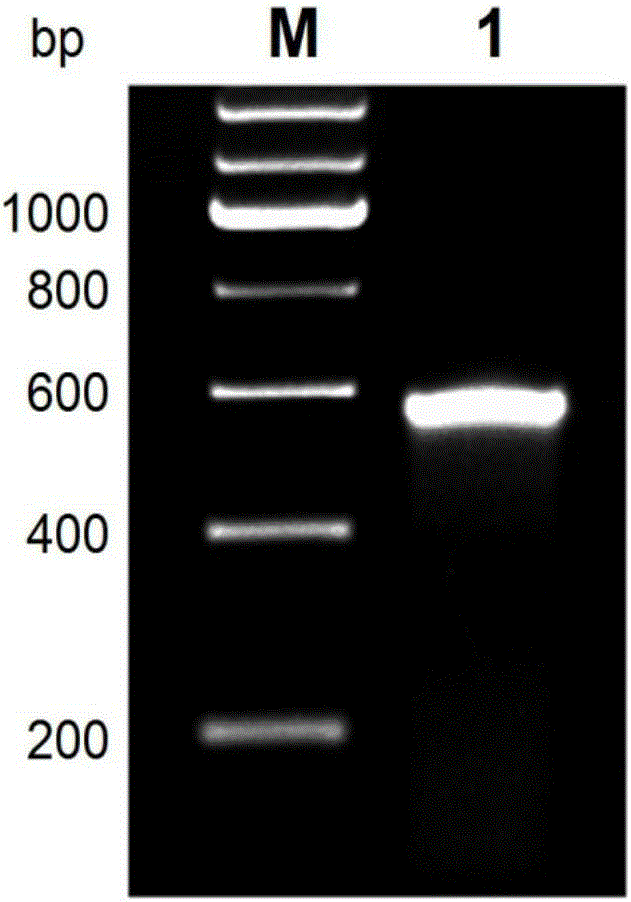
B19 virus VP1 unique region gene
The invention discloses a B19 virus VP1 unique region gene, and constructs a B19 procaryon to express pQE30-VP1 unique region of cloning plasmid, express and purify the recombinant protein; the recombinant protein can cause immune cells response in vitro, and produce the highly effective antibodies; the B19 virus recombination protein of the present invention can be used for antigen diagnosis and for preparing antibody and vaccine for prevention of B19 virus.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Establishment method of animal model in mice infected with sparganosis mansoni
InactiveCN101926307AAvoid high cross-reactivityStrong specificityIn-vivo testing preparationsAnimal husbandryAntigenQuarantine
The invention discloses an establishment method of an animal model in mice infected with sparganosis mansoni, comprising the following steps: cleaning and soaking polypide taken out from the body of the animal naturally infected with sparganum mansoni with sterilized normal saline; injecting the polypide into the gullets of the healthy mice by the method for pouring belly for inoculation; after raising the inoculated mice for a period, transplanting the inoculated mice by the method for pouring belly, carrying out standard raising on the transplanted mice and carrying out one-time new rejuvenation transplantation and collection on the infected mice which are raised for a period within 3-12 months; and establishing the stable animal model in mice infected with sparganum mansoni. The method specifies the collection method of the sparganum mansoni species resources and the living body conservation operation technology, provides the stable disease animal model for the research on immunity characteristics, immunopathological injury, diagnosis and treatment of sparganosis mansoni and provides the standard species resources with stable biological characteristics for the scientific research, teaching, quarantine and diagnostic antigen production units.
Owner:ZHENGZHOU UNIV
Toxoplasma indirect hemagg lutination diagnostic reagent and producing process thereof
The insect indirect blood diagnosis regent and its making method are made by the antigen secreted by the bow form insect. It inoculates the 1*10-5-1*10-6 bow form insect into the small rat belly cavity, then to soak in the alcohol to disinfect the body surface after making the small white rat death to collect a belly cavity liquid by the centrifugation; the red cell treated by the secreted antigen which is the bow insect indirect blood diagnosis antigen; the 5% red cell treated by the secreted antigen are suspended in the water bath box, then to centrifugate and wash for 3-5 times; then to wash by the 2% NRS of 0.15M PBS with the pH7.2 for 2-3 times. the sediments is to matched to the 1% sensitized red cell suspension with the 0.15M PBS of pH7.2 containing 2% NRS. It is dried by the freeze drier and vacuumize to get the indirect blood diagnosis regent with the IHA secreted by the bow form insect. The invention can be used for examining the bow form insect disease and the census aspect of bow form insect disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Schistosoma japonicum katsurada recombinant protein SjSAPLP5 as well as encoding gene and application thereof
ActiveCN105254732AImproving immunogenicityGood antigenicityBiological material analysisAntiparasitic agentsAntigenDisease
The invention provides a schistosoma japonicum katsurada recombinant protein SjSAPLP5 as well as an encoding gene and an application thereof. The protein provided by the invention has amino acid sequence as shown in SEQ ID No.2 or has amino acid sequence which is formed by replacement, deletion and / or addition of one or more of amino acid residues and has the same function. The invention also provides the encoding gene for encoding the protein. The recombinant protein SjSAPLP5 provided by the invention is excellent in immunogenicity, can be served as an excellent diagnosis antigen, is used for preparing a schistosoma japonicum katsurada diagnostic kit with high sensitivity and high specificity, and is also used for preparing an anti-schistosome vaccine and the drug served as a potential drug effecting target spot and is used for screening and treating the schistosoma japonicum katsurada diseases.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Application of sarcoptes protein tyrosine kinase and kit for diagnosing sarcoptic acariasis
ActiveCN107478835AStrong reactogenicity100% detection rateBiological material analysisSerum igeAntigen
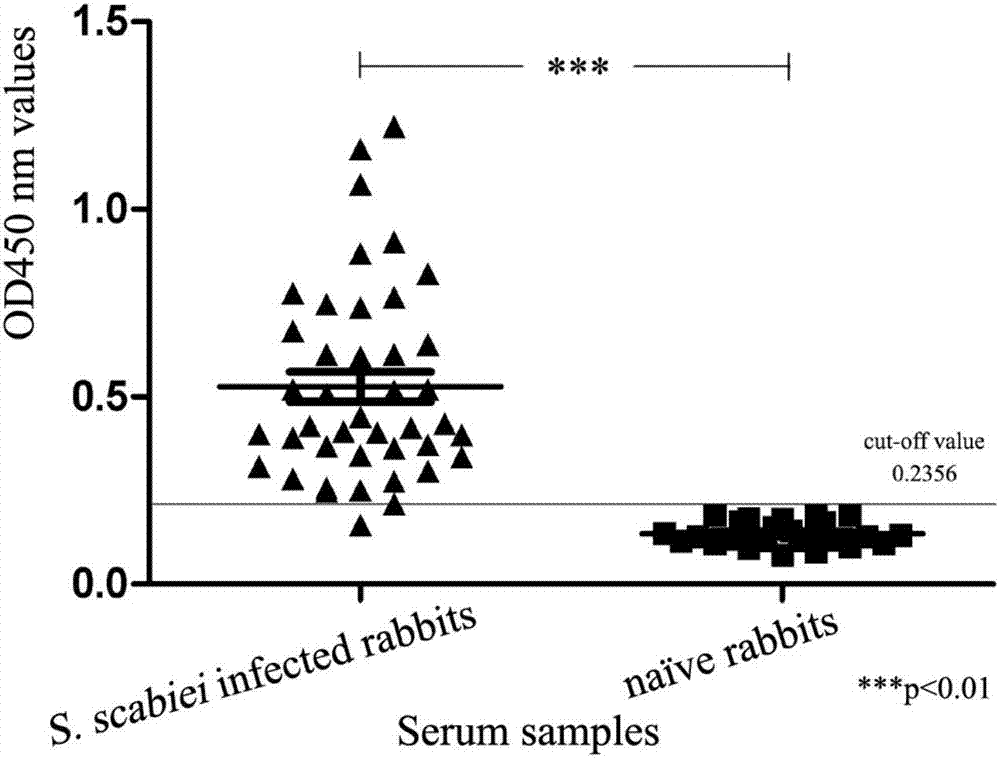
The invention relates to the technical field of biology, and discloses the application of sarcoptes protein tyrosine kinase serving as a sarcoptic acariasis diagnostic antigen. Relevant experimental results show that the sarcoptes protein tyrosine kinase can be recognized by rabbit positive serum of naturally infected sarcoptes mites and anti-PTK rabbit serum, and has high reactogenicity, an SsPTK-ELISA method shows up extremely high sensibility and specificity, has one hundred percent of detection rate of early sarcoptic acariasis, and various results prove that the sarcoptes protein tyrosine kinase can serve as a diagnostic antigen of sarcoptic acariasis particularly in the diagnosis of early sarcoptic acariasis.
Owner:SICHUAN AGRI UNIV
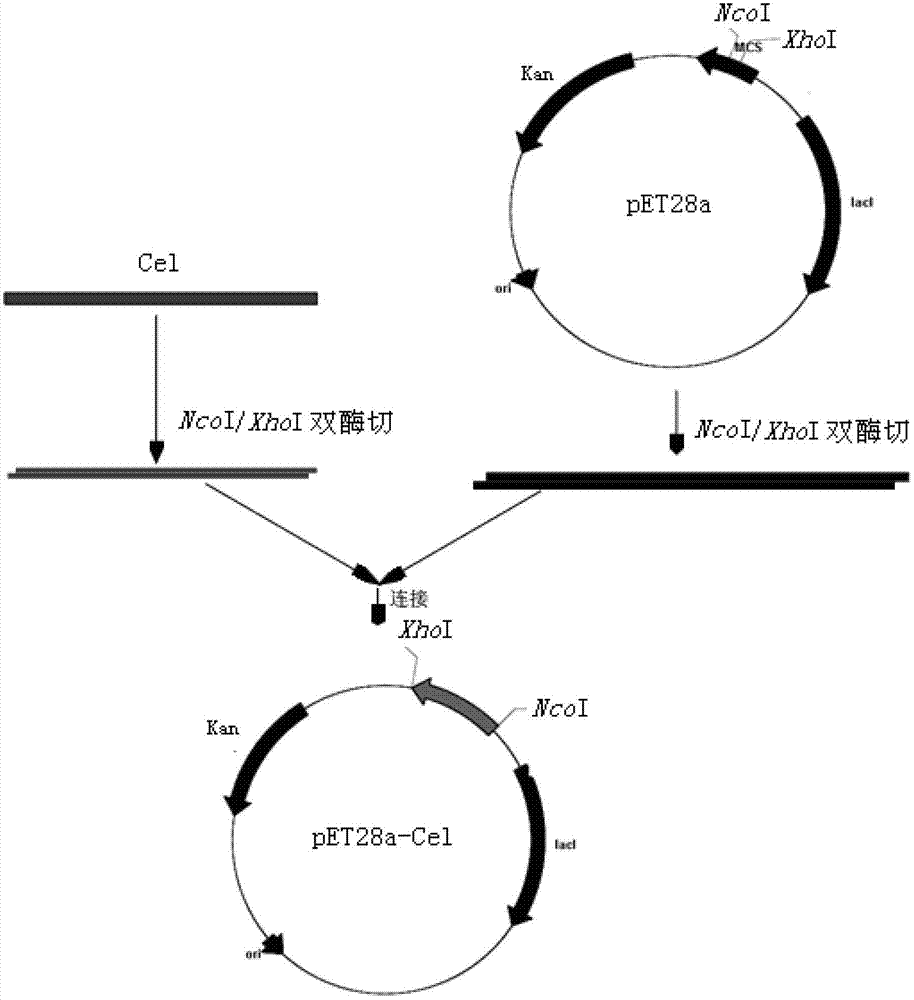
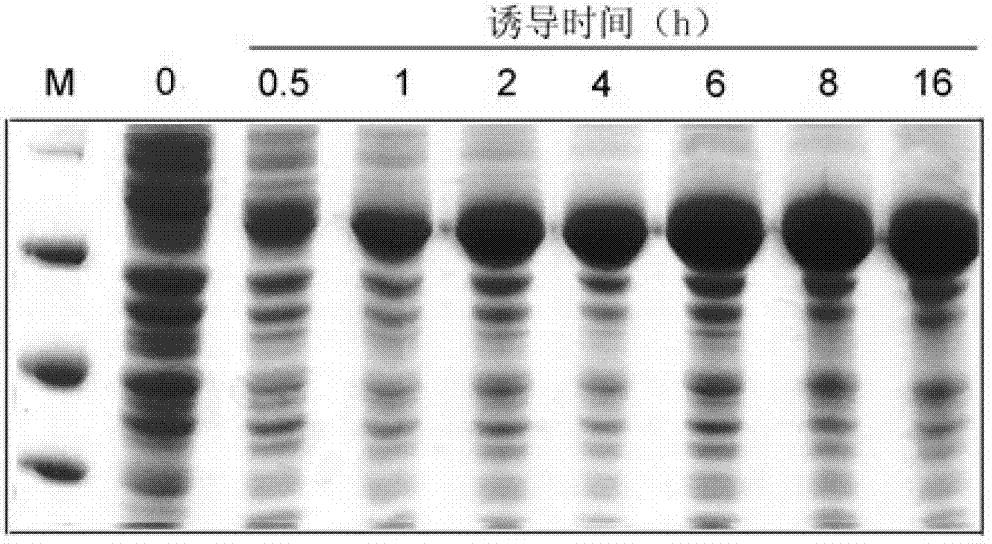
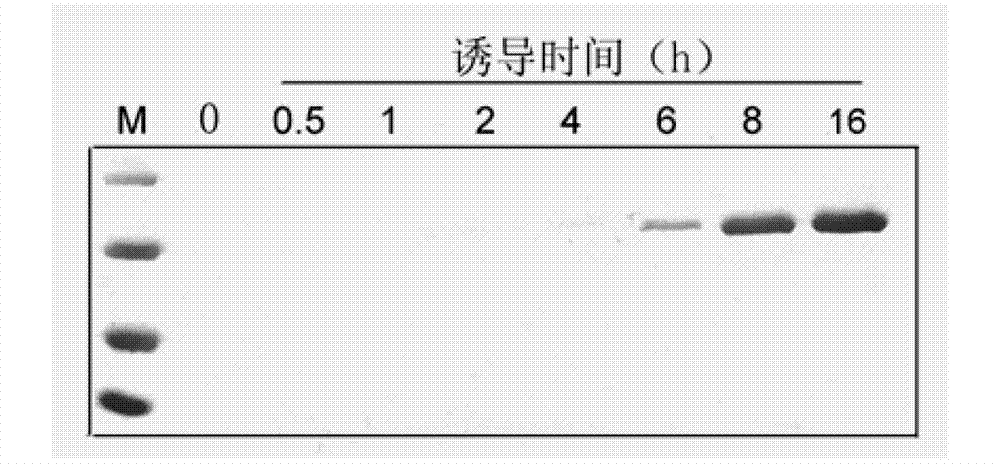
Application of cellulase in soluable and secretion expression of recombinant protein in escherichia coli
ActiveCN102776216APreferred separation and purification methodHydrolasesAnimals/human peptidesEscherichia coliADAMTS Proteins
The invention discloses an application of cellulase in soluable and secretion expression of recombinant protein in escherichia coli. The protein sequence is an amino acid sequence represented by SEQ ID NO: 1-7, or a polypeptide sequence having 70% or more homology with the amino acid sequence represented by SEQ ID NO: 1-7, and the recombinant protein is antibacterial peptides or enzymes. The application of cellulase in soluable and secretion expression of recombinant protein in escherichia coli provides a foundation for preparing protein / polypeptide drugs, various enzymes, diagnostic antigens, antibodies, vaccines and the like by using escherichia coli, and has important theoretical meanings and application values in protein expression study, enzymic preparations, protein drug production and the like.
Owner:SHANDONG UNIV
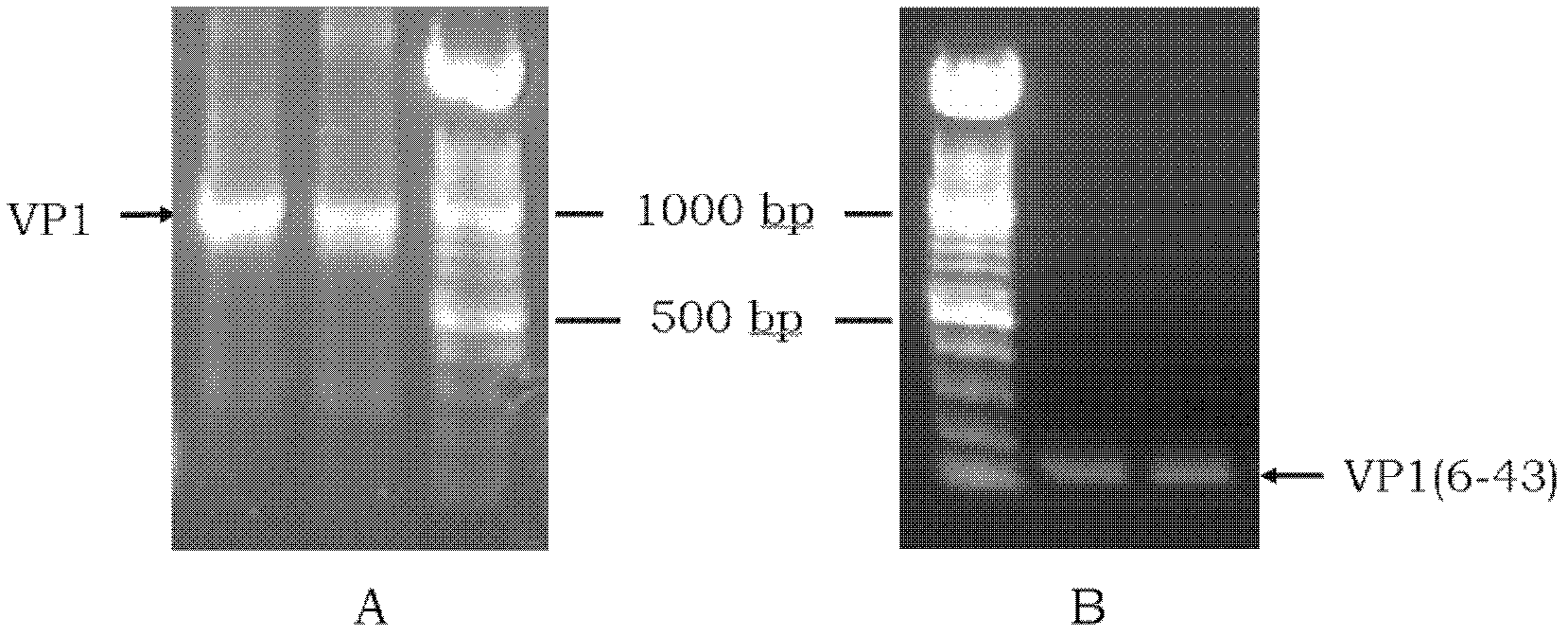
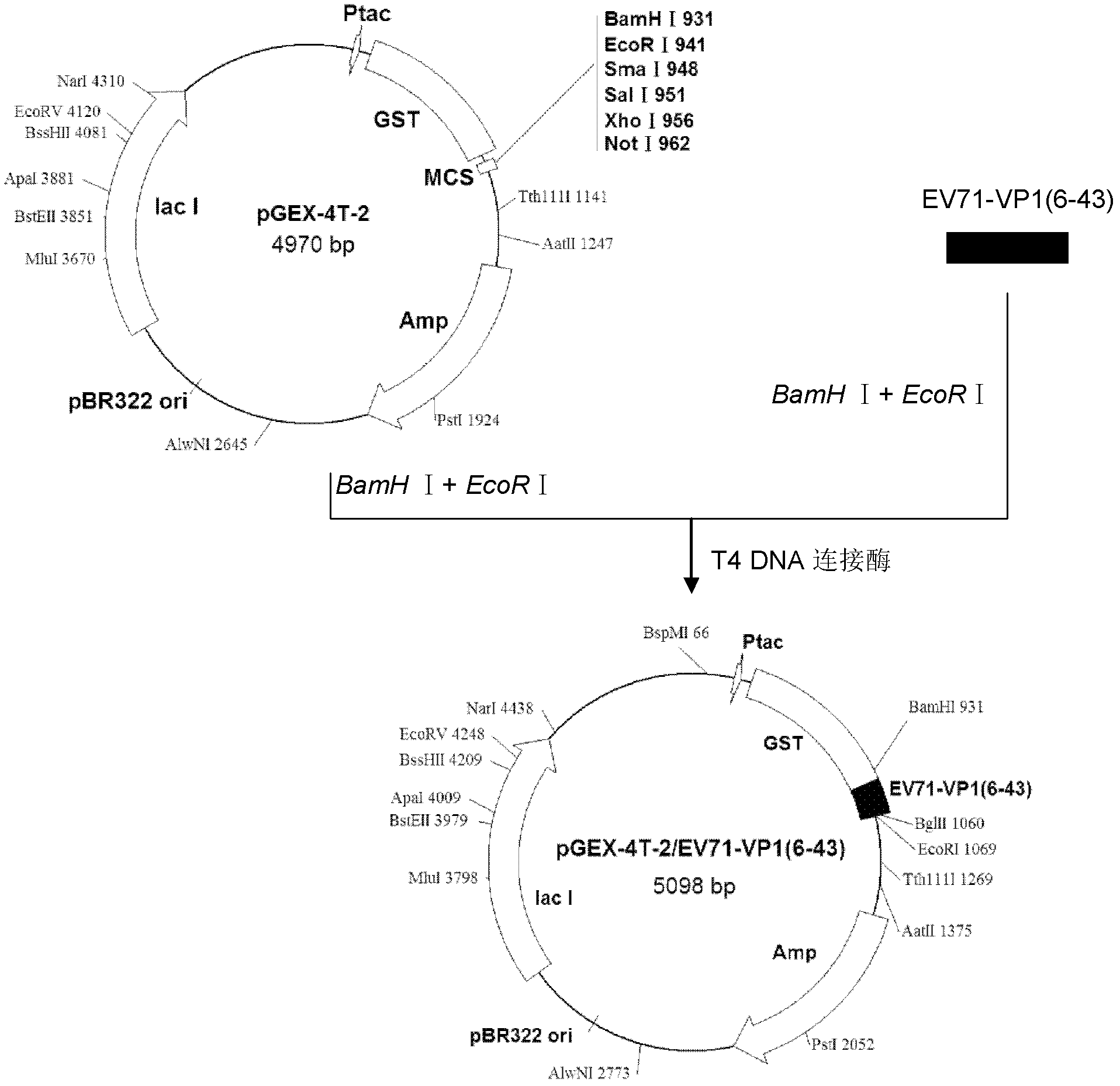
Method for purifying recombinant VP1 antigen of enterovirus type 71 viruses
ActiveCN101857871AHigh recovery rateImprove renaturation yieldPeptide preparation methodsDepsipeptidesEnterovirusWater baths
The invention relates to a method for purifying a recombinant protein of the gene engineering and aims to provide a method for purifying a recombinant VP1 antigen of enterovirus type 71 viruses. The method comprises the following steps of: expressing and producing a recombinant VP1 protein of the enterovirus type 71 viruses by Escherichia coli obtain an inclusion body of the protein; dissolving the inclusion body in solubilizing liquid; and dropwise adding the solution of the inclusion body into renaturation solution under the conditions of water bath and magnetic stirring and then carrying out stirring renaturation on the mixture for 48 hours. The invention discloses the method, which can make the protein existing in a form of the inclusion body become a soluble protein by protein renaturation so as to greatly improve recovery rate of an expression product. The method adopts affinity chromatography for purification in one step. The purity is over 95 percent. The method for purifying the recombinant VP1 antigen of the enterovirus type 71 viruses has simple, convenient and time-saving operation, is favorable for large-scale industrial production, has the advantages of low cost, simple and convenient process, high purity of the purified protein and the like and can provide the diagnostic antigen for development of an EV type 71 diagnostic reagent and seroepidemiological survey.
Owner:HANGZHOU ZHEDA ZIJIN BIOTECH
Methods and compositions for detecting larval taenia solium with a cloned diagnostic antigen
InactiveUS20040033540A1Inexpensive and sensitive and accurateLittle or no cross-reactivityBacterial antigen ingredientsProtozoa antigen ingredientsAntigenTaenia solium
Compositions and methods for the detection of Taenia solium and the diagnosis of T. solium infection are described. The nucleotide and amino acid sequences of the antigenic T. solium polypeptides gp50a, gp50b and gp50c are provided. The compositions contain synthetic antigenic polypeptides of larval origin prepared using the sequences described herein. Probes and primers for the detection or amplification of T. solium nucleic acid molecules are also described. The polypeptides can be administered to a human or animal to protect against T. solium infection. In addition, the polypeptides are useful as research tools for studying T. solium and as reagents in assays for the detection of T. solium antibodies in a biological sample. The methods are sensitive and specific assays that utilize the stable recombinant or synthetic antigenic polypeptides or nucleic acid molecules encoding the larval polypeptides.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Babesia caballi disease immunoblotting detection method and preparation of kit
The invention discloses a babesia caballi disease immunoblotting detection method and a kit. The babesia caballi disease immunoblotting detection method is established on the basis of obtaining a diagnostic antigen and positive control serum through a goat anti horse second antibody compound marked by horse radish peroxidase and a chemiluminescence substrate. The method has the advantages of less reagent dosage, low cost, high specificity, high sensitivity, quicker operation, no toxicity and harm, low required condition, easy judgment of a test result, long-term storage and stronger specificity than ELISA (Enzyme-Linked Immuno Sorbent Assay). By detecting by using the method, false positive and false negative results are not detected.
Owner:中华人民共和国天津出入境检验检疫局
Computer vision method for improved automated image capture and analysis of rapid diagnostic test devices
ActiveUS11494571B2Improve accuracyHigh sensitivityHealth-index calculationCharacter and pattern recognitionRapid screening testAntigen
The disclosed embodiments are generally directed to improving feature detection of rapidly acquired images using camera-enabled mobile devices involving a 2-D decal code, such as a QR code, for improving the reading accuracy of a rapid diagnostic antigen or antibody or enzymatic colorimetric directed test, such as for COVID-19 diagnosis. One primary issue with evaluating a Covid-19 rapid test is detecting and quantifying positive test lines from sampled test strips based on digital images of the test strip. Aspects of the present invention contemplate masking a QR code to improve the sample image resolution and contrast. Other aspects of the present invention contemplate methods and techniques to evaluate a test line on the sample image by enhancing an intensity curve along the test line and control line containing area by way of calculating the instantaneous change in pixel intensity and evaluating the position and intensity of those signals.
Owner:NEUROGANICS DIAGNOSTICS LLC
Pseudorabies virus GE gene major antigen-epitope region recombinant protein preparation and colloidal-gold immunochromatographic strip
InactiveCN110540578ASmall sample sizeEasy to detectVirus peptidesBiological material analysisAntigen epitopeAntigen
The invention provides a pseudorabies virus GE protein and preparation of a colloidal-gold immunochromatographic strip thereof. The pseudorabies virus colloidal-gold immunochromatographic strip is provided with a carrier plate, a sample pad, a colloidal-gold conjugate pad, a laminate membrane, a detection line, a quality-control line and an absorption pad. The expressed recombinant protein existedin a supernatant is easy to purify. The detection method established by taking the recombinant protein, which is purified by affinity chromatography, as a diagnostic antigen is low in cost and simpleand convenient, and especially the diagnostic antigen concentrates major antigen-epitopes of the gE antigen. The detection sensitivity and specificity of the recombinant protein are both higher thanthe intact gE protein.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com