Method for purifying recombinant VP1 antigen of enterovirus type 71 viruses
A purification method and enterovirus technology, applied in the field of genetic engineering recombinant protein purification, can solve the problems of low protein recovery rate, low protein expression amount, long production cycle and the like, and achieve the effects of high purity, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1. Construction and fermentation of recombinant EV71 VP1 engineering bacteria
[0039] The VP1 gene is derived from the C4 subtype of enterovirus type 71. In this example, the whole gene of VP1 was designed and synthesized with the preferred codons of Escherichia coli by using a total synthesis method. The ADI gene was double digested with Nde I and BamH I, embedded in the same digested vector pET3a to construct the vector pET3a-VP1, transformed into host cell BL21 DE3 (pLyss), and positive clones were screened and sequenced. The positive strains with correct sequencing were used as the original engineering bacteria for production. Pick a single clone and culture in 500ml LB liquid medium containing 50ug / ml ampicillin antibiotic at 37°C with shaking at 300rpm until OD 600 Reach about 1.2, inoculate into 15L fermenter (B.Braun C) until OD 600 When it reached about 20 (double distilled water was used as blank control), 1mM IPTG was added to induce expression. ...
example 2
[0040] Example 2. Renaturation
[0041] The fermentation broth was centrifuged at 6000 g for 15 minutes to collect the bacteria. The cells were broken by high-pressure homogenization at 1500 psi, and the inclusion bodies were collected by centrifugation at 6000 g for 15 minutes, and the inclusion bodies were washed twice with PBS buffer. Dissolve according to the ratio of 1g inclusion body: 15ml increasing solution, the increasing solution composition is 6M guanidine hydrochloride, 5mM EDTA, 10mM Tris, pH 8.5, stirred at 25°C for 2 hours. Slowly pour into 2000ml refolding solution (10mM PB, 100mM Arg, 10% PEG20000, pH 7.2). Stir at 15°C for renaturation for 48 hours.
example 3
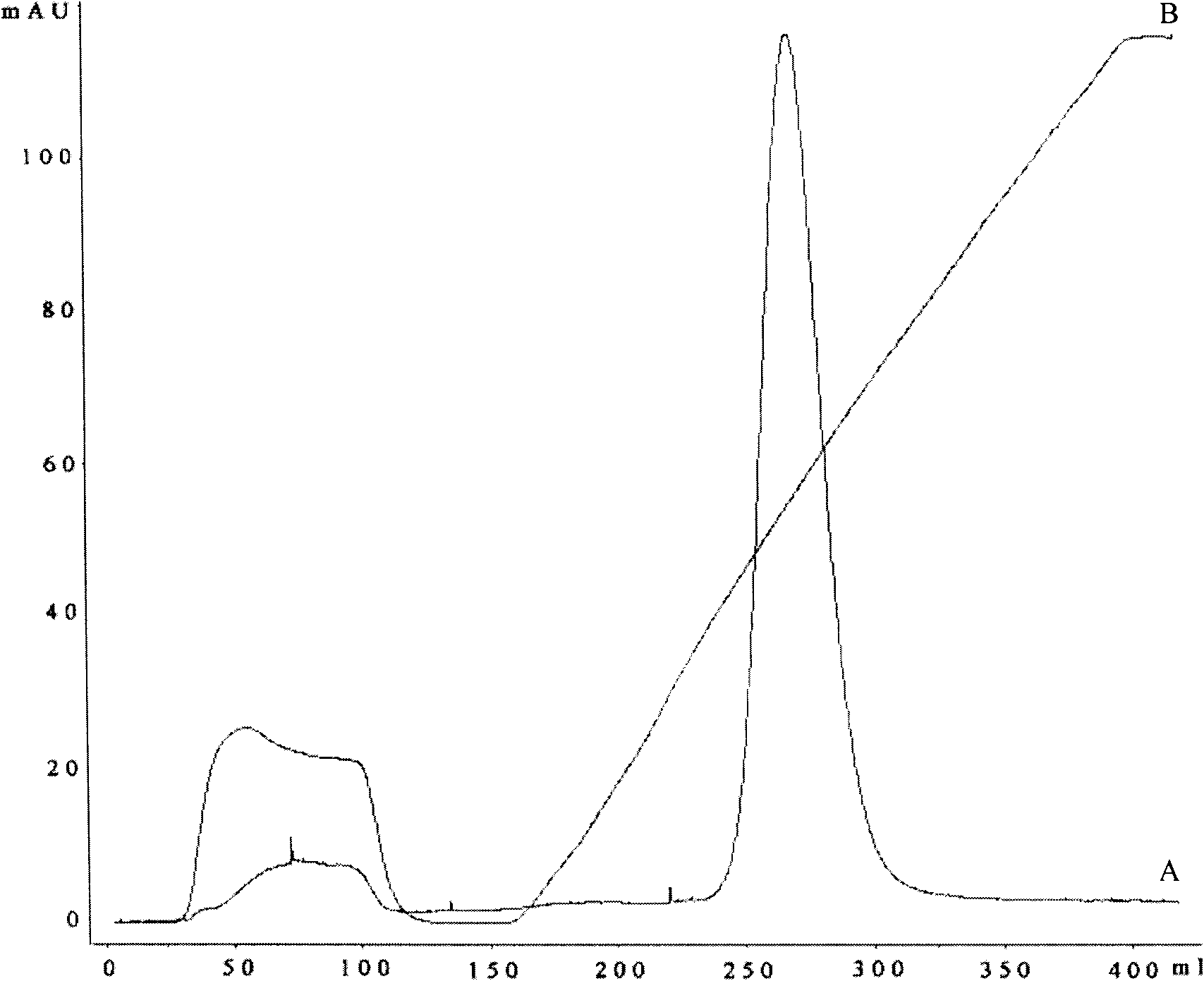
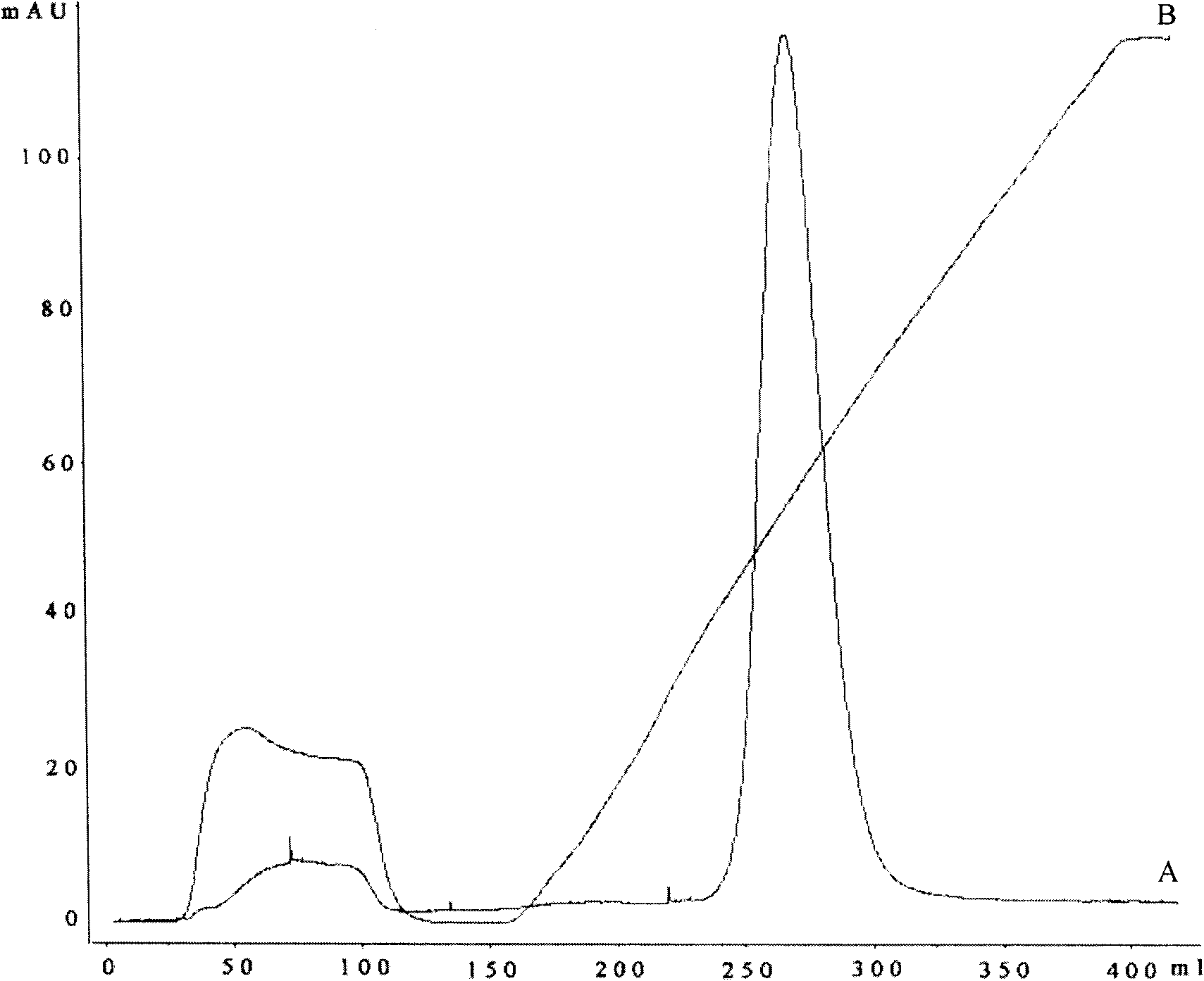
[0042] Example 3. Purification
[0043] 200mL of Chelating Sepharose Fast FlowSepharose gel was loaded into the XK 50 / 30 column of GE Company, the equilibration buffer was 20mM PB, 0.5M NaCl, pH 7.0, the elution buffer was 20mM PB, 0.5M NaCl, 0.5M imidazole, pH 7.0. Equilibrate the column first with 0.2M nickel sulfate solution and then with equilibration buffer. The refolding solution was loaded onto the equilibrated column at a flow rate of 20ml / min. After the sample was loaded, the equilibrated buffer was used to wash 2 column volumes, and then eluted with a linear gradient to collect the target peak. SDS-PAGE electrophoresis was used to detect the purity of the target protein, and the Lowry method was used to detect the target protein concentration. After analysis, the amount of VP1 protein that can be obtained per gram of inclusion body is 200 mg, the protein recovery rate is 20%, and the purity is more than 95% as checked by SDS-PAGE electrophoresis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com