Schistosoma japonicum katsurada recombinant protein SjSAPLP5 as well as encoding gene and application thereof
A recombinant protein, schistosomiasis technology, applied in recombinant DNA technology, application, genetic engineering and other directions, to achieve the effect of good immunogenicity and good antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
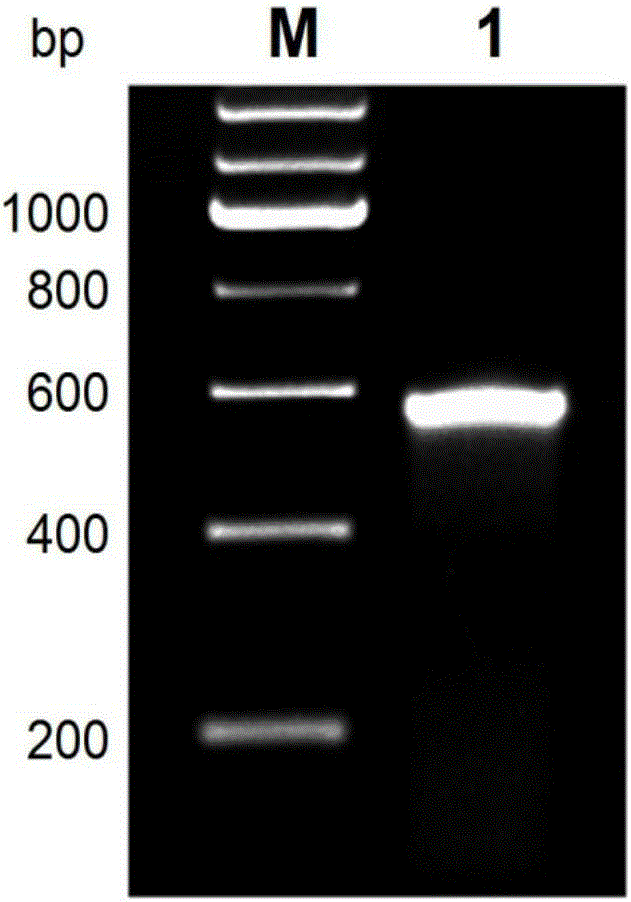
[0045] Example 1 Cloning of SjSAPLP5 gene of Schistosoma japonicum
[0046] According to the sequence of SjSAPLP5 gene (GenBankFN315317.1) (as shown in SEQIDNO.1), design primers and introduce restriction sites, as follows:
[0047] PF: 5’-CGC GGATCC TCAACATTGAACAGTAATAAT-3' (as shown in SEQ ID NO.3, the italicized part represents the protective base of the upstream primer, and the underlined part is the BamHI restriction site of the upstream primer);
[0048] PR: 5’-CTG CTCGAG TGCAAAAGGATATTTCAAA-3' (as shown in SEQ ID NO.4, the italicized part represents the protective base of the downstream primer, and the underlined part is the XhoI restriction site of the downstream primer);
[0049] The specific primers were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.
[0050] Using the cDNA of 42-day male and female adults of Schistosoma japonicum as a template, PCR was performed to amplify the ORF fragment of SjSAPLP5 gene. The reaction system was as follows: high-fidelity DNA polym...
Embodiment 2
[0054] Example 2 Expression and purification of SjSAPLP5 recombinant protein of Schistosoma japonicum
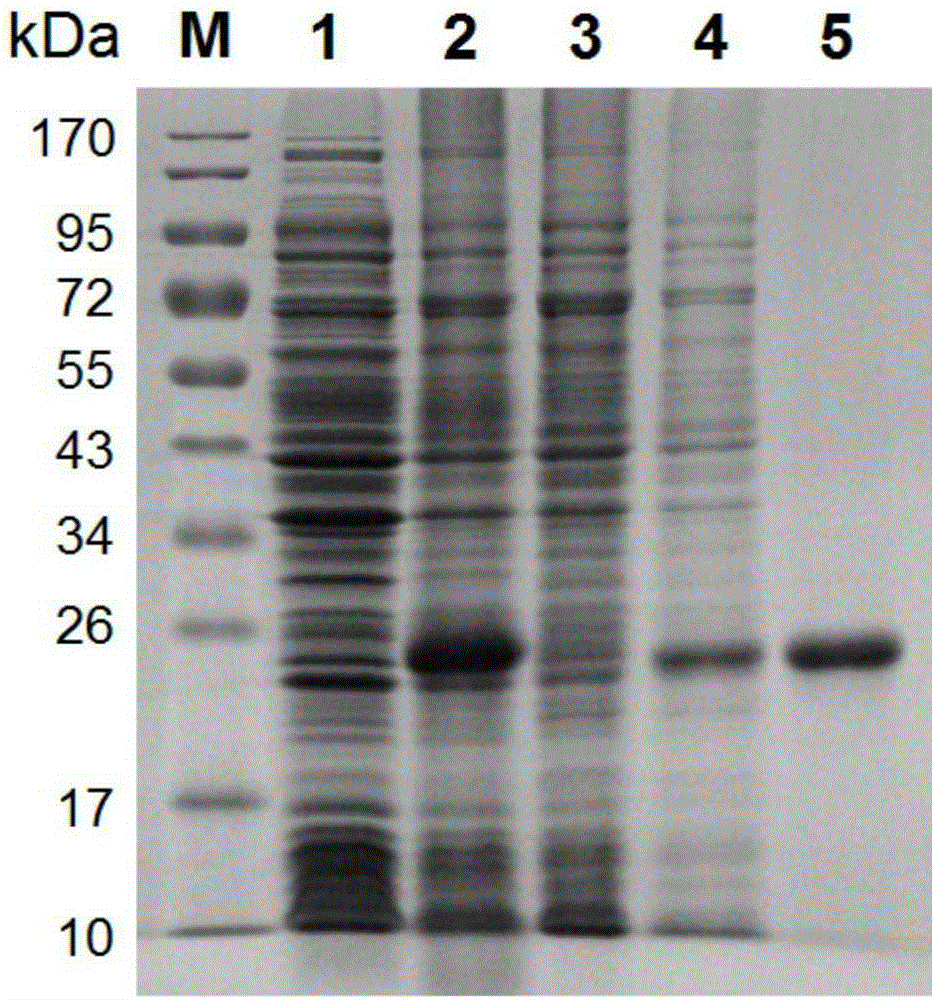
[0055] Transform the correctly sequenced pET28a(+)-SjSAPLP5 recombinant plasmid to express competent cells Transetta(DE3) (Beijing Quanshijin Biotechnology Co., Ltd.); inoculate the positive clones by PCR into LB liquid medium (containing 50μg) / ml kanamycin) 15mL, incubate overnight at 37°C, transfer 10mL medium to 1L of LB medium (containing 50μg / ml kanamycin) the next day, and continue to cultivate to OD 600nm The value was 0.8, and IPTG with a final concentration of 1 mM was added to induce expression for 4 hours, and the cells were collected by centrifugation, and frozen at -80°C for use.
[0056] Suspend a small amount of bacteria before and after induction in PBS buffer, add SDS-PAGE loading buffer, mix well and cook for 5 minutes in a boiling water bath to denature the protein. Add 10 μl of the sample before and after induction to each sample well, and perform SDS-PAGE a...
Embodiment 3
[0058] Example 3 Antigenicity detection of SjSAPLP5 recombinant protein of Schistosoma japonicum
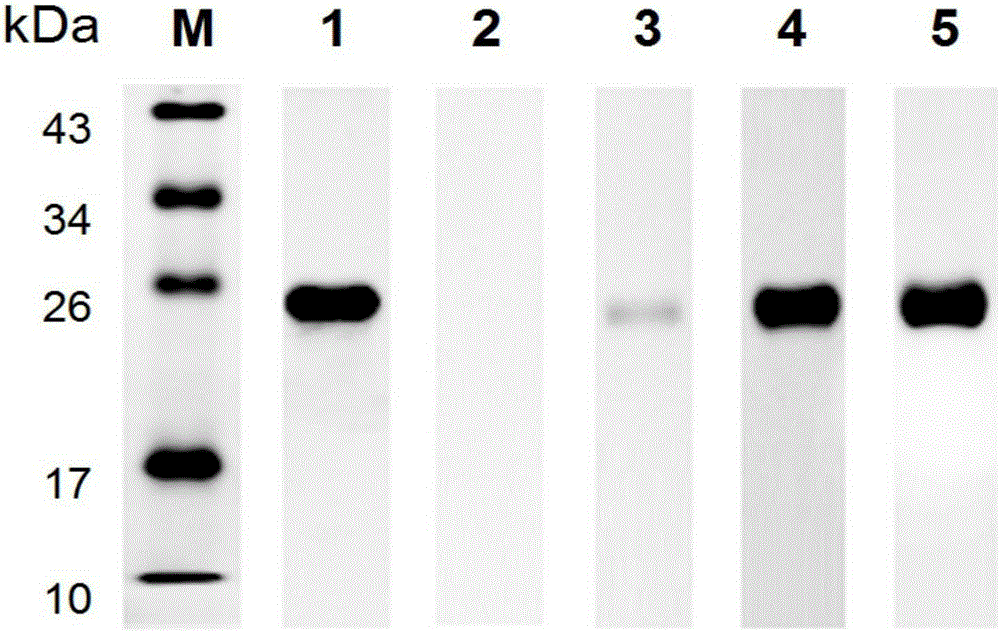
[0059] SDS-PAGE electrophoresis: 100ng of the SjSAPLP5 recombinant protein obtained in Example 2 was loaded and the electrophoresis conditions were: 100V20min, 120V1h.
[0060] Transfer membrane: Use the wet transfer method to transfer the protein in the PAGE gel to the PVDF membrane. The electrotransfer conditions are: ice bath, 100V1h.
[0061] Blocking: Block the PVDF membrane with 5% skimmed milk powder at room temperature for 2 hours, and wash 3 times with TBST buffer.
[0062] Incubation with primary antibody: add the serum of BALB / c mice infected with Schistosoma japonicum for 42 days, New Zealand white rabbit serum from Schistosoma japonicum for 42 days, and the serum of Schistosoma japonicum patients, with mouse anti-His-Tag antibody (Abimart Biomed Company) as a positive control, healthy mouse serum as a negative control (diluted with blocking solution 1:500), incubated overnig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com