Ilepcimide-containing controlled release tablet as well as preparation method and application thereof
A technology of controlled-release tablets and content, which is applied in the field of medicine, can solve the problems of poor patient compliance, high blood drug concentration side effects, poor children's compliance, etc., reduce the number of times and doses taken, reduce the fluctuation of blood drug concentration, and improve drug use. safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
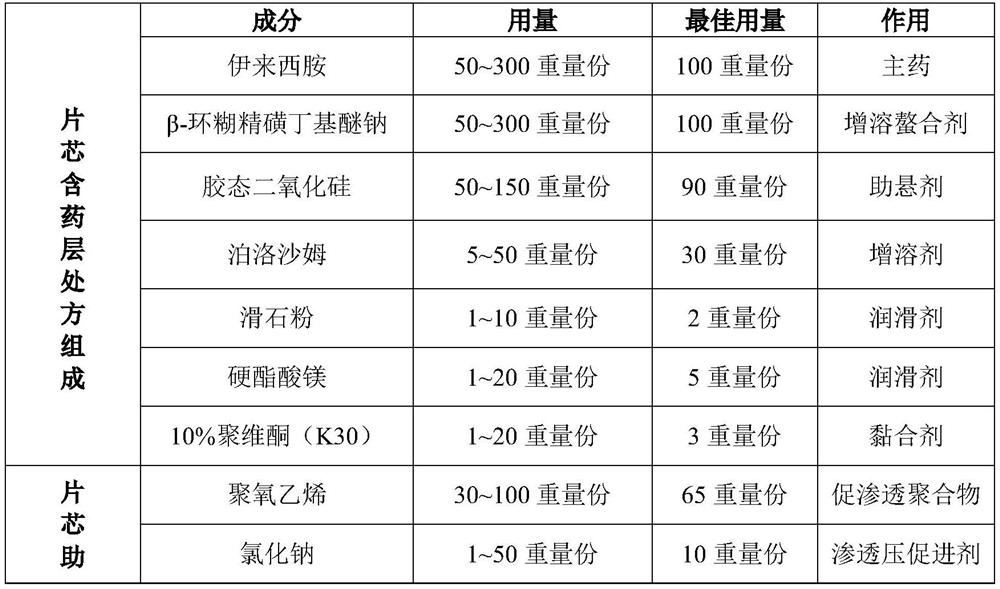
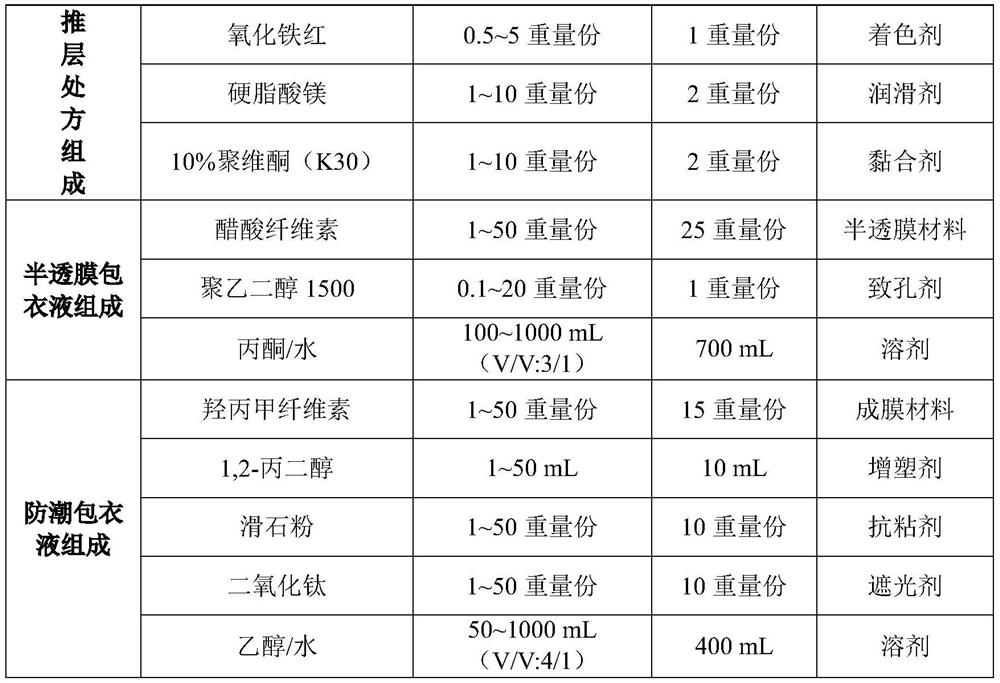
[0146] prescription:
[0147]
[0148]
[0149] Preparation Process:
[0150] (1) Preparation of chelates: pulverizing the ileximide and crossing a 100-mesh sieve;
[0151] Weigh β-cyclodextrin sulfobutyl ether sodium according to the prescription amount, and add it into water according to the mass ratio of 2:1 to obtain an aqueous solution of β-cyclodextrin sulfobutyl ether sodium; Mix the sodium butyl ether aqueous solution, stir evenly, filter, dry, and sieve through a 100-mesh sieve to obtain the sodium butyl ether chelate (ie, ECH-(SBE)7m-β-CD ,1:2), spare.
[0152] (2) drug-containing layer: mix eleximide-β-cyclodextrin sodium sulfobutyl ether chelate with colloidal silicon dioxide and poloxamer passed through a 60-mesh sieve, mix evenly according to the prescription amount, add mass The soft material is made from an aqueous solution of povidone with a fraction of 10%, granulated with a 24-mesh sieve, dried at 45°C for 12 hours, sized with a 24-mesh sieve, then ...
Embodiment 2
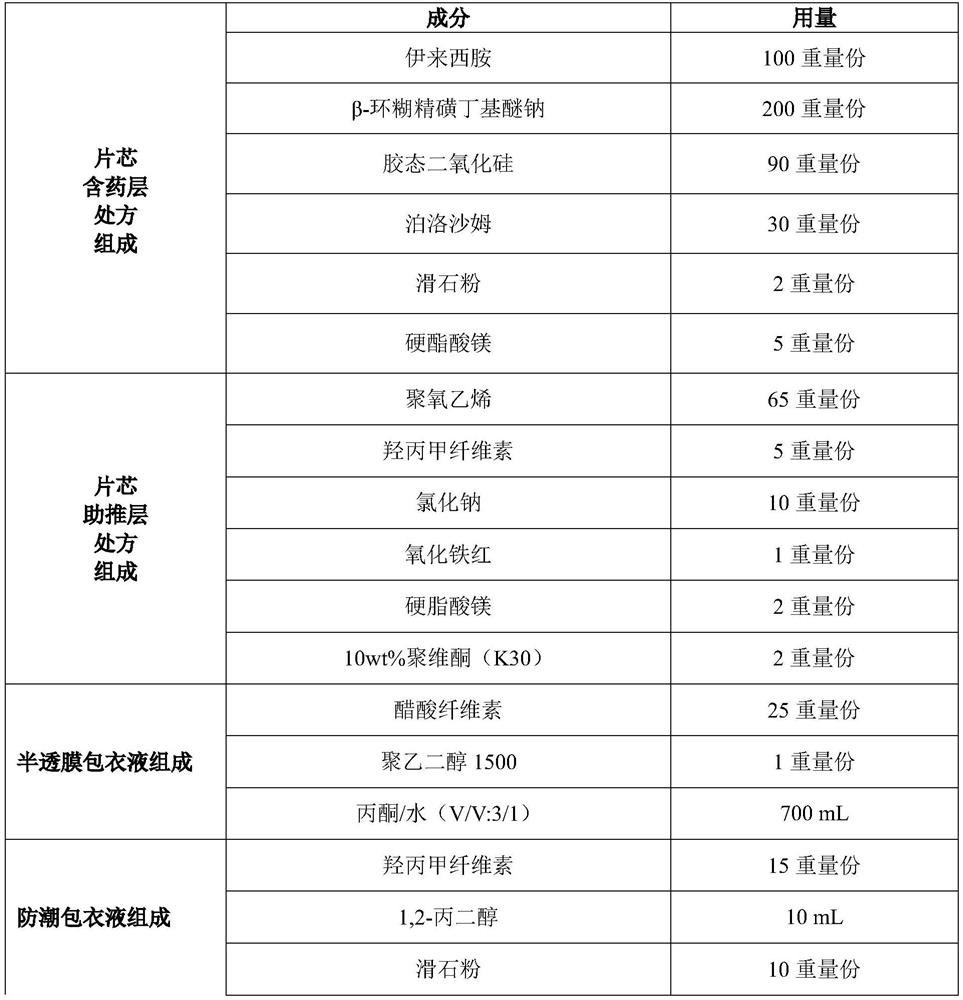
[0156] prescription:
[0157]
[0158]
[0159] Preparation Process:
[0160] (1) Preparation of chelates: pulverizing the ileximide and crossing a 100-mesh sieve;
[0161] Weigh according to the prescription amount, add β-cyclodextrin sulfobutyl ether sodium into water at a mass ratio of 1.5:1 to obtain an aqueous solution of β-cyclodextrin sulfobutyl ether sodium; add elexinamine to β-cyclodextrin Mix the aqueous solution of sodium sulfobutyl ether, stir evenly, filter, dry, and sieve through a 100 mesh sieve to obtain the sodium sulfobutyl ether chelate (ie ECH-(SBE)7m-β- CD,1:1.5), spare.
[0162] (2) drug-containing layer: mix eleximide-β-cyclodextrin sodium sulfobutyl ether chelate with colloidal silicon dioxide and poloxamer passing through a 60-mesh sieve, mix evenly according to the prescription amount, add mass Fractionally add 10% povidone aqueous solution to make a soft material, granulate with a 24-mesh sieve, dry at 45°C for 12 hours, granulate with a 24...
Embodiment 3
[0166] prescription:
[0167]
[0168] Preparation Process:
[0169] (1) Preparation of chelates: pulverizing the ileximide and crossing a 100-mesh sieve;
[0170] Weigh according to the prescription quantity, add β-cyclodextrin sulfobutyl ether sodium into water at a mass ratio of 1:1 to obtain an aqueous solution of β-cyclodextrin sulfobutyl ether sodium; add elexinamine to β-cyclodextrin Mix the aqueous solution of sodium sulfobutyl ether, stir evenly, filter, dry, and sieve through a 100 mesh sieve to obtain the sodium sulfobutyl ether chelate (ie ECH-(SBE)7m-β- CD,1:1), spare.
[0171] (2) drug-containing layer: mix eleximide-β-cyclodextrin sodium sulfobutyl ether chelate with colloidal silicon dioxide and poloxamer passed through a 60-mesh sieve, mix evenly according to the prescription amount, add mass The soft material is made from an aqueous solution of povidone with a fraction of 10%, granulated with a 24-mesh sieve, dried at 45°C for 12 hours, sized with a 24-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com